DESCRIPTIVE PROPERTIES OF Matter A EXTENSIVE VS INTENSIVE




- Slides: 8

DESCRIPTIVE PROPERTIES OF Matter

A. EXTENSIVE VS. INTENSIVE Extensive Property §depends on the amount of matter present Intensive Property §depends on the identity of substance, not the amount

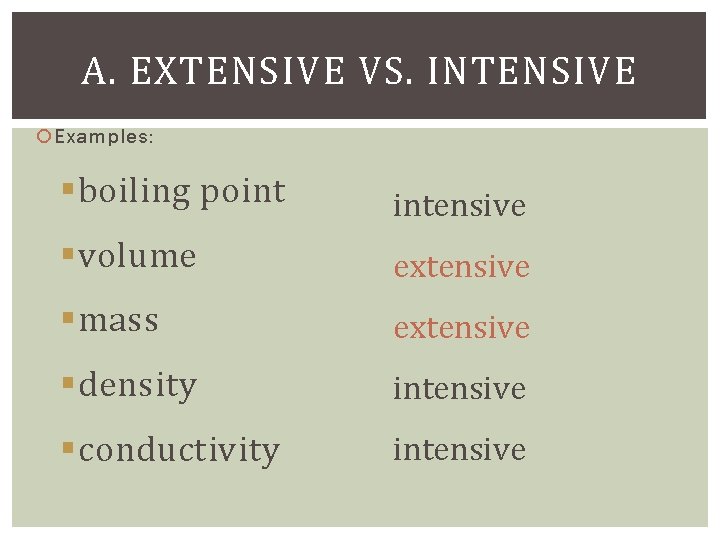
A. EXTENSIVE VS. INTENSIVE Examples: §boiling point intensive §volume extensive §mass extensive §density intensive §conductivity intensive

DENSITY Mass g What are the units for each? Density g/m. L = Volume m. L Types of Values • Accepted Value – the ‘book’ value, what the value is supposed to be. • Experimental Value – the value found in the lab.

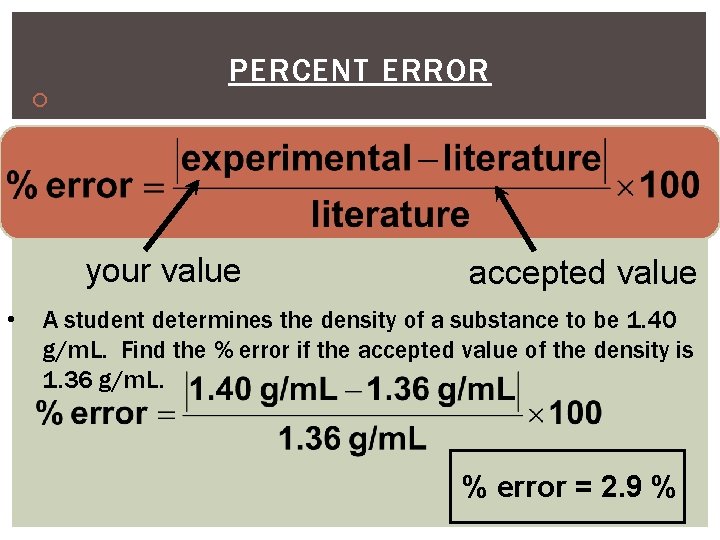
PERCENT ERROR Indicates accuracy of a measurement your value • accepted value A student determines the density of a substance to be 1. 40 g/m. L. Find the % error if the accepted value of the density is 1. 36 g/m. L. % error = 2. 9 %

CLASSIFICATION OF MATTER Mass is the amount of matter the object contains. Matter is anything that has a mass and volume. Volume is the amount of space an object takes up. Examples of things that are not matter: § Light and energy Picture a Golf Ball and a Ping Pong Ball… Which has more mass? More volume?

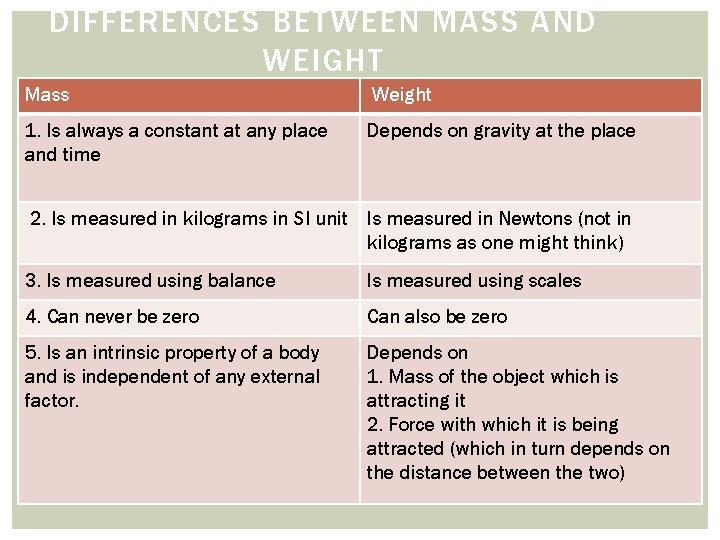
DIFFERENCES BETWEEN MASS AND WEIGHT Mass Weight 1. Is always a constant at any place and time Depends on gravity at the place 2. Is measured in kilograms in SI unit Is measured in Newtons (not in kilograms as one might think) 3. Is measured using balance Is measured using scales 4. Can never be zero Can also be zero 5. Is an intrinsic property of a body and is independent of any external factor. Depends on 1. Mass of the object which is attracting it 2. Force with which it is being attracted (which in turn depends on the distance between the two)

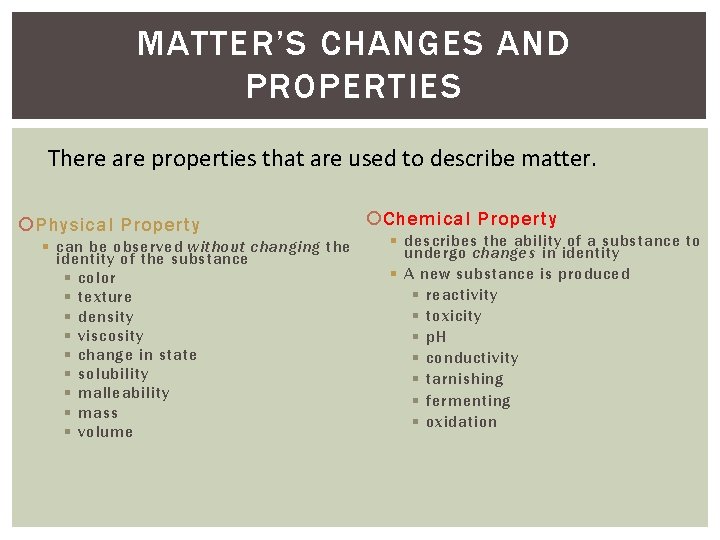
MATTER’S CHANGES AND PROPERTIES There are properties that are used to describe matter. Physical Property § can be observed without changing the identity of the substance § color § texture § density § viscosity § change in state § solubility § malleability § mass § volume Chemical Property § describes the ability of a substance to undergo changes in identity § A new substance is produced § reactivity § toxicity § p. H § conductivity § tarnishing § fermenting § oxidation