MATTER Definition of matter Properties of matter Classification












- Slides: 18

MATTER ØDefinition of matter. ØProperties of matter. ØClassification of matter. ØPhysical states of matter. ØPhysical changes in matter. ØChemical changes in matter.

WHAT IS MATTER? Matter is everything around us that has mass and takes up space. Matter can be solid, liquid or gas. Rocks, living things, water and air are examples of matter.

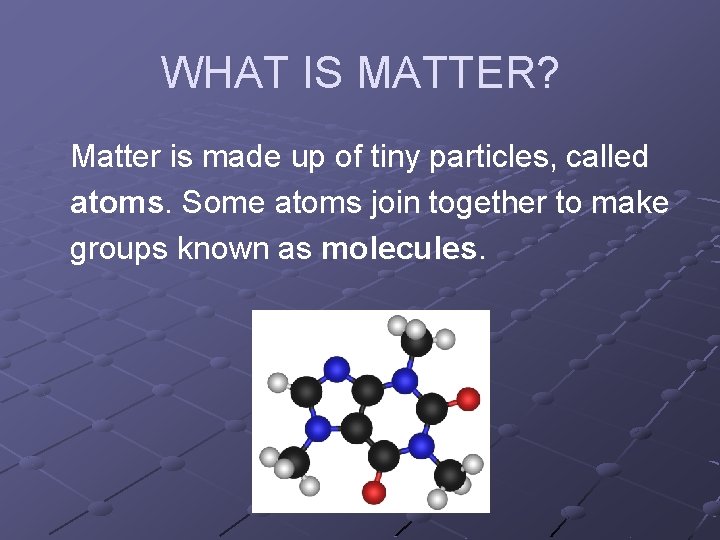
WHAT IS MATTER? Matter is made up of tiny particles, called atoms. Some atoms join together to make groups known as molecules.

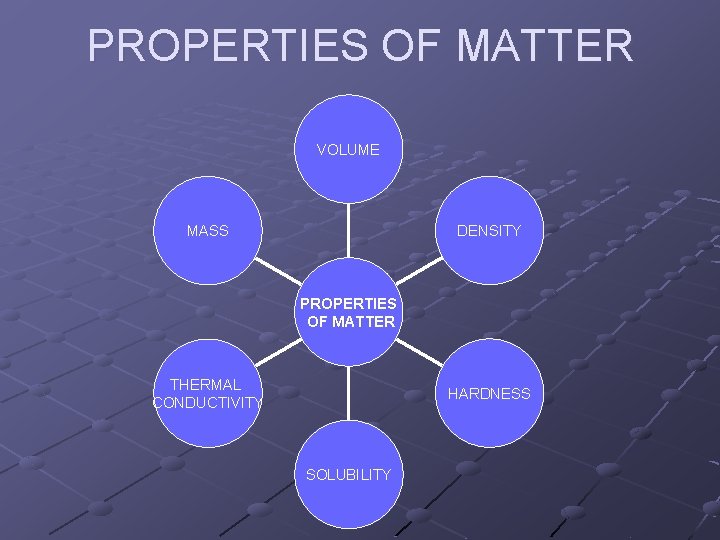
PROPERTIES OF MATTER VOLUME DENSITY MASS PROPERTIES OF MATTER THERMAL CONDUCTIVITY HARDNESS SOLUBILITY

PROPERTIES OF MATTER MASS Mass is the amount of matter in an object. We measure mass in grams or kilograms. An apple has greater mass than a grape.

PROPERTIES OF MATTER VOLUME Volume is the amount of space an object occupies. Bigger objects have more volume than smaller objects. We measure volume in litres or mililitres. A car has larger volume than a motorcycle.

PROPERTIES OF MATTER THERMAL CONDUCTIVITY Thermal conductivity is the ability of certain objects to conduct or transfer heat. Most metals are good heat conductors.

PROPERTIES OF MATTER SOLUBILITY Solubility is the ability of a substance to dissolve in other substance and form a solution. Sugar dissolves well in water, whereas oil does not.

PROPERTIES OF MATTER HARDNESS Hardness is the scratch-resistance of a solid. Diamonds are the hardest natural solids.

DENSITY PROPERTIES OF MATTER Density is the amount of matter in a volume. We measure density in kilograms per litre (Kg/l) density= mass/volume Density explains why some objects float in water while others sink.

DENSITY PROPERTIES OF MATTER Does it float or does it sink? Why? cork water iron density 0, 25 kg/l 1, 00 kg/l 7, 90 kg/l Cork has a lower density than water. It floats! Iron has a higher density than water. It sinks!

CLASSIFICATION OF MATTER According to its composition, matter can be classified into pure substances and mixtures. Ø Pure substances are made up of only one type of matter. Ø Mixtures are made up of two or more pure substances.

Silver or table salt are examples of pure substances.

Heterogeneous mixture Homogeneous mixture Sand or chocolate milk are examples of mixtures.

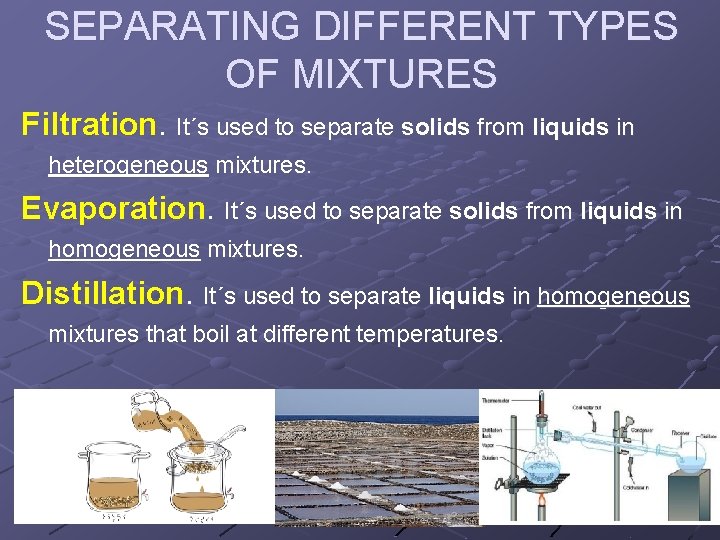
SEPARATING DIFFERENT TYPES OF MIXTURES Filtration. It´s used to separate solids from liquids in heterogeneous mixtures. Evaporation. It´s used to separate solids from liquids in homogeneous mixtures. Distillation. It´s used to separate liquids in homogeneous mixtures that boil at different temperatures.

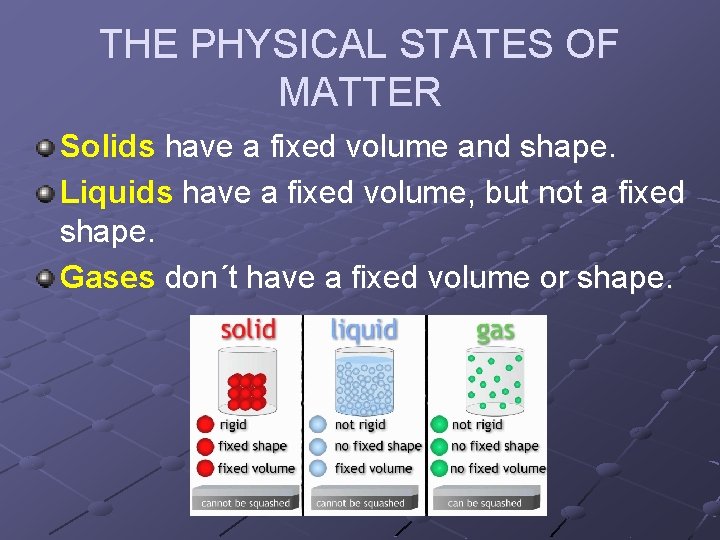
THE PHYSICAL STATES OF MATTER Solids have a fixed volume and shape. Liquids have a fixed volume, but not a fixed shape. Gases don´t have a fixed volume or shape.

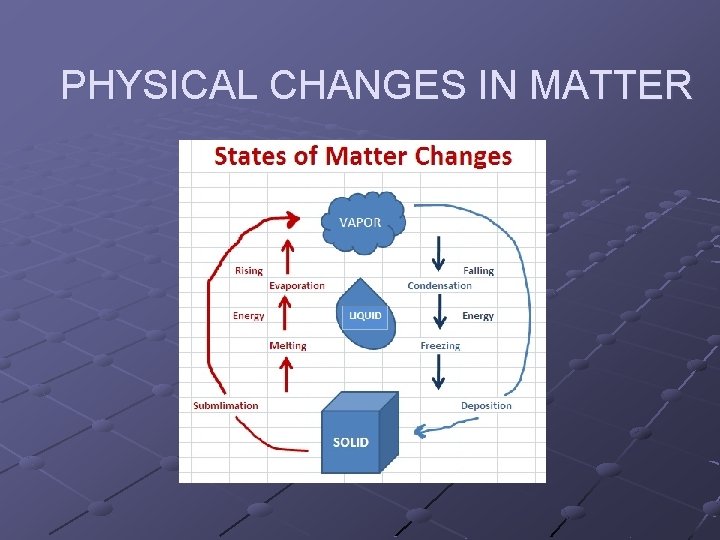
PHYSICAL CHANGES IN MATTER

CHEMICAL CHANGES IN MATTER A new substance is produced. àOxidation. When substances combine with oxigen. It produces rust àCombustion. When objects or substances are burned. It produces smoke and ashes.