Properties of Matter Physical Properties Chemical Properties Physical















- Slides: 21

Properties of Matter

• Physical Properties • Chemical Properties

Physical Properties • • • Mass Color Melting Point Boiling Point Density State (at normal conditions)

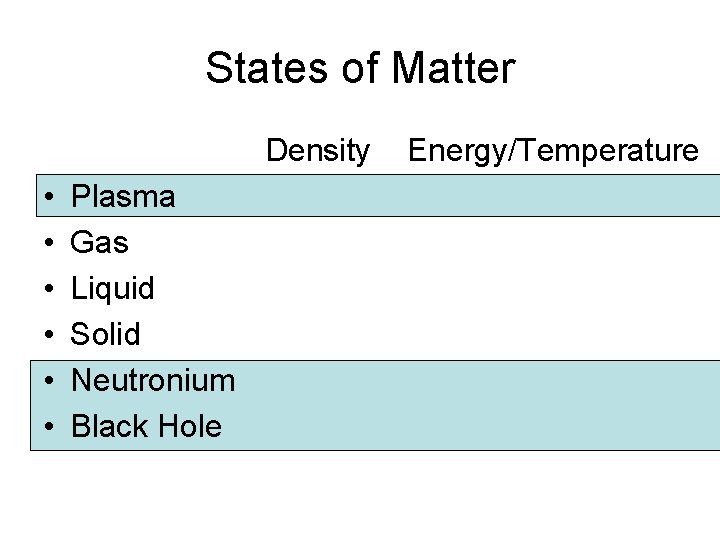
States of Matter Density • • • Plasma Gas Liquid Solid Neutronium Black Hole Energy/Temperature

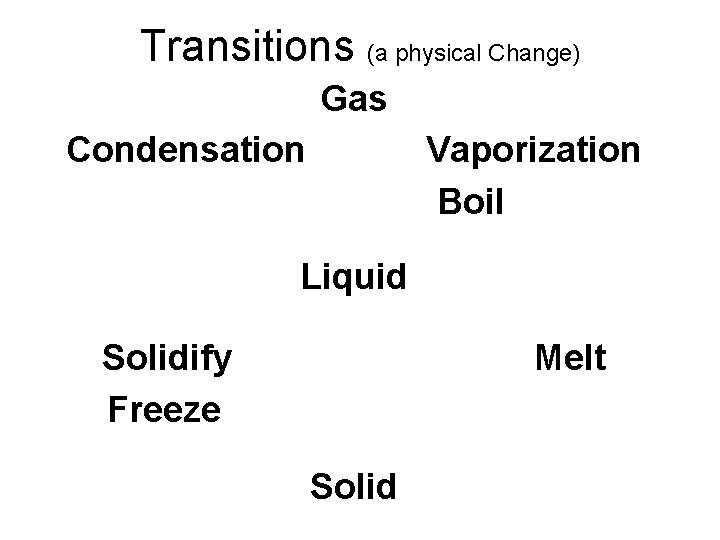
Transitions (a physical Change) Gas Condensation Vaporization Boil Liquid Solidify Freeze Melt Solid

• Physical Change

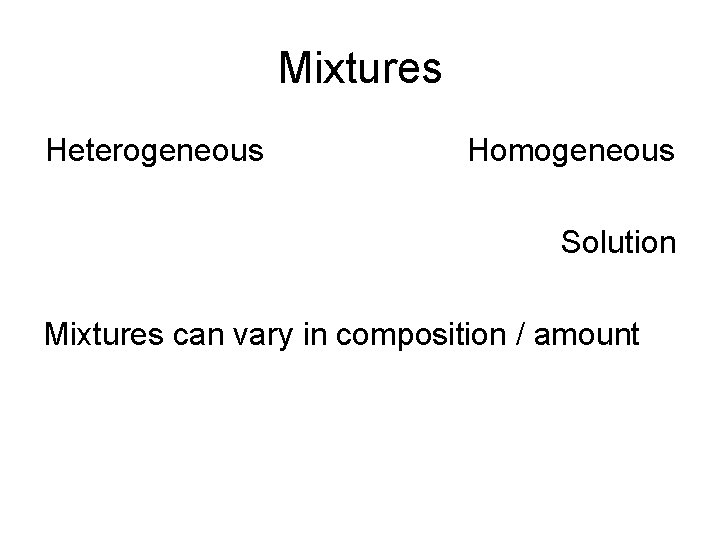
Mixtures Heterogeneous Homogeneous Solution Mixtures can vary in composition / amount

Separation of Mixtures • Using Physical Methods / Physical Properties • • Salt and pepper Salt and sand Water and salt Water and Alcohol – distillation


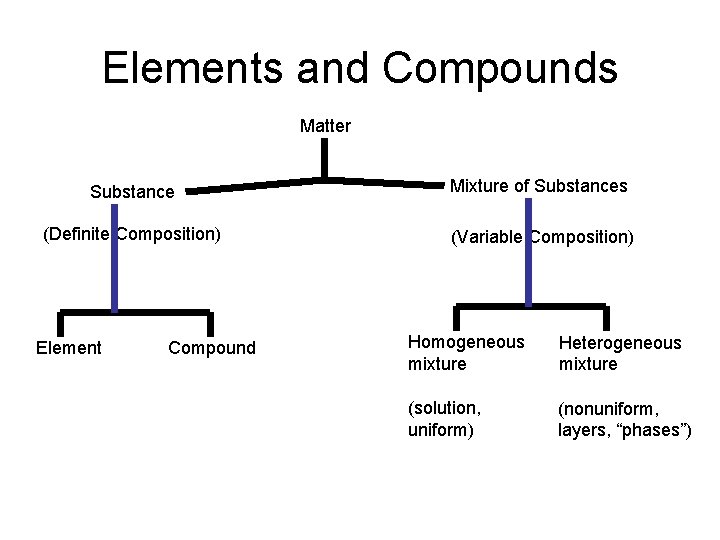
Elements and Compounds Matter Substance Mixture of Substances (Definite Composition) (Variable Composition) Element Compound Homogeneous mixture Heterogeneous mixture (solution, uniform) (nonuniform, layers, “phases”)

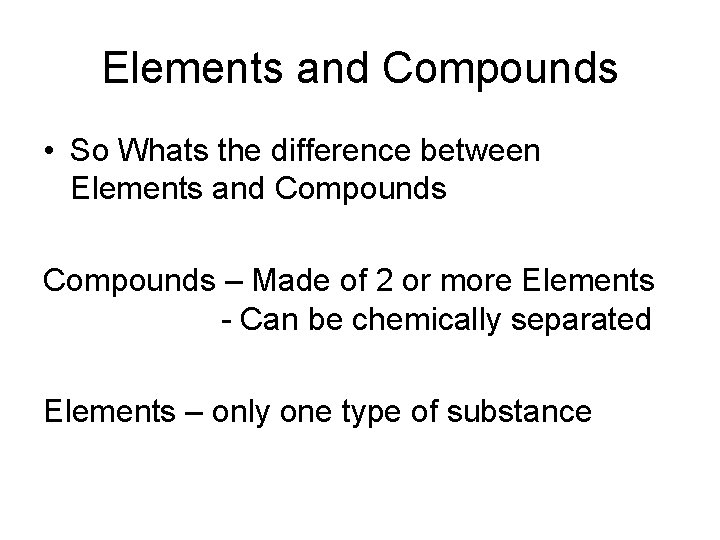
Elements and Compounds • So Whats the difference between Elements and Compounds – Made of 2 or more Elements - Can be chemically separated Elements – only one type of substance

• Is it important? ? ? • Well, getting some definitions straight is good… • And, it lets you know what kinds of things might go on… • Like, what happens when you had Sodium and Chlorine – or Sodium to Water?

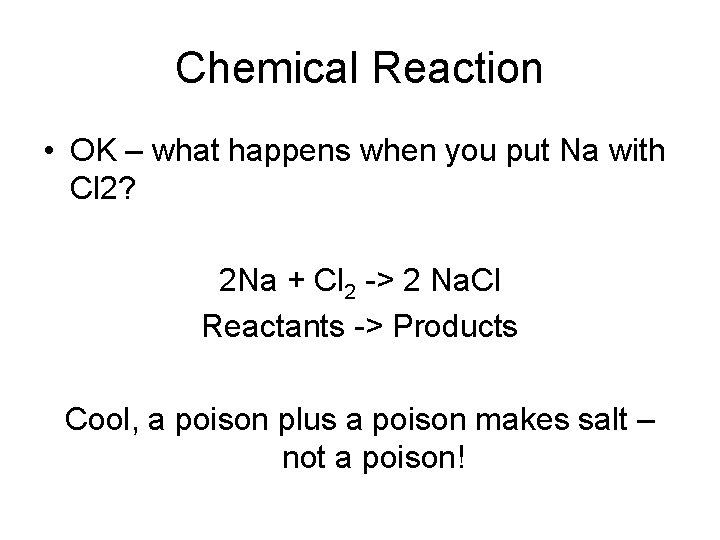
Chemical Reaction • OK – what happens when you put Na with Cl 2? 2 Na + Cl 2 -> 2 Na. Cl Reactants -> Products Cool, a poison plus a poison makes salt – not a poison!



How do you know it’s a Chemical Reaction? • Energy is Absorbed (cools) or Released (gives off heat) • Light! (sound) • Color change • Gas formed, Solid formed (precipitate) • Not easily reversible • (are there exceptions to these… well, yes)

Conservation of Mass Conservation of Energy • In a chemical reaction* – matter cannot be created nor destroyed • In a chemical reaction* - Energy cannot be created nor destroyed

Hold on – what about atomic bombs • OK – you got me – the REAL answer is • Matter and energy can neither be created nor destroyed – it can only change forms.

Baker - @ Bikini - RMI


Disclaimer Aloha I put together these power points for use in my science classes. You may use them in your classes. Some images are public domain, some are used under the fair-use provisions of the copyright law, some are mine. Copyright is retained by the owners! Ted Brattstrom
 Chemical and physical properties
Chemical and physical properties True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Measurable properties of matter
Measurable properties of matter What are chemical properties of matter
What are chemical properties of matter Physical properties of matter jeopardy
Physical properties of matter jeopardy Graphic organizer matter
Graphic organizer matter Chemical properties of notebook paper
Chemical properties of notebook paper Chemical properties of sulphuric acid
Chemical properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Thermal conductivity in dentistry
Thermal conductivity in dentistry Physical and chemical properties
Physical and chemical properties Hardness property of matter
Hardness property of matter Physical properties of oxygen family
Physical properties of oxygen family Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Physical and chemical properties interactive
Physical and chemical properties interactive Chemical and physical properties of silver
Chemical and physical properties of silver Chemical properties of maltose
Chemical properties of maltose Physical property
Physical property Water chemical and physical properties
Water chemical and physical properties Physical and chemical properties sorting activity
Physical and chemical properties sorting activity Section 1 composition of matter
Section 1 composition of matter White matter nervous system
White matter nervous system