Physical and Chemical Properties Physical Properties Are properties



















- Slides: 25

Physical and Chemical Properties

Physical Properties � Are properties that do NOT change the chemical nature of matter � They are usually what we can observe with our senses. (sight, smell, hear, touch, taste) �A substance’s physical properties allow us to identify the substance without causing a change in the composition of the substance.

Lustre � How shiny or dull a material is � Example Silver has high lustre vs. Chalk is dull

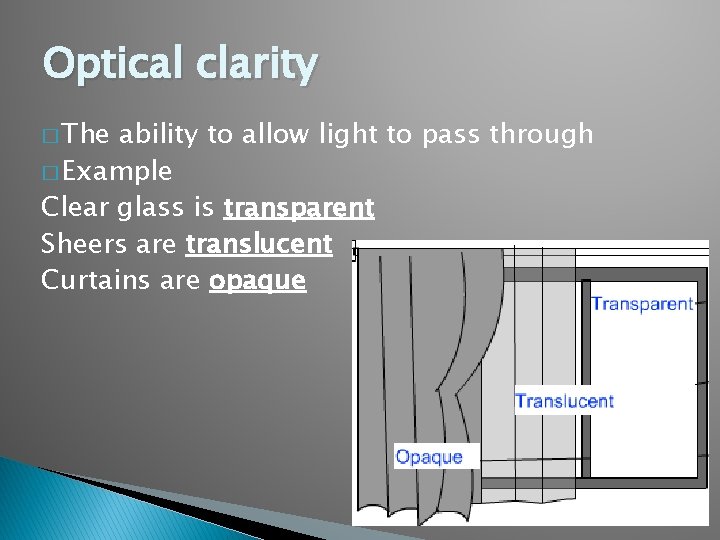
Optical clarity � The ability to allow light to pass through � Example Clear glass is transparent Sheers are translucent Curtains are opaque

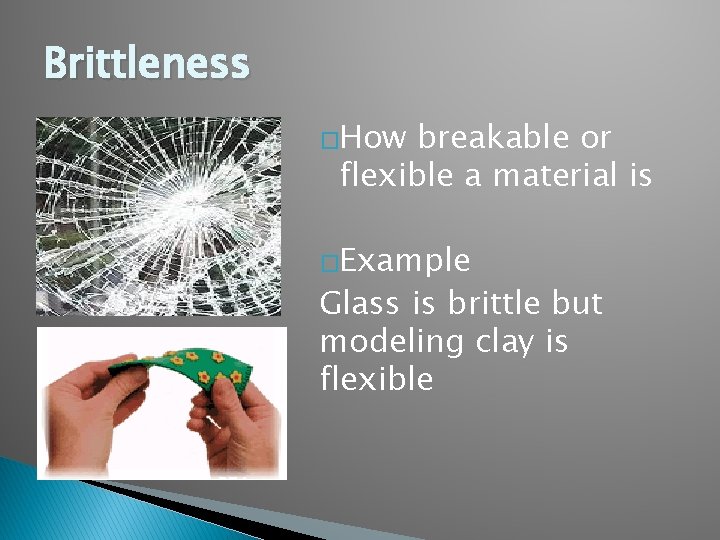
Brittleness �How breakable or flexible a material is �Example Glass is brittle but modeling clay is flexible

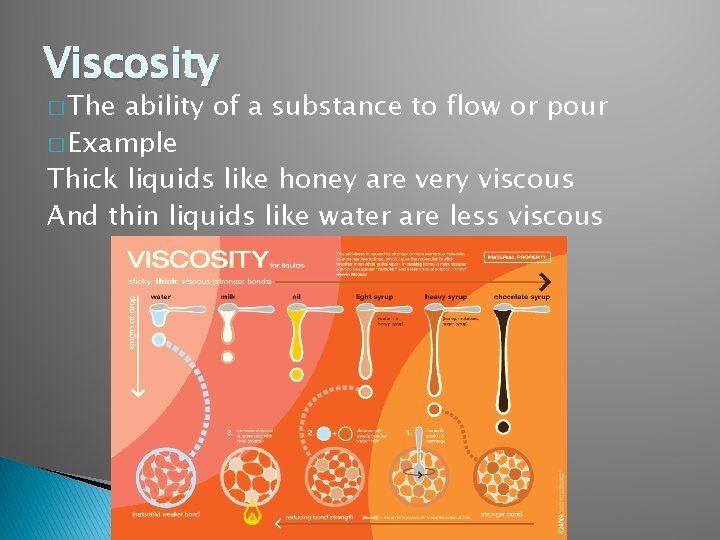
Viscosity � The ability of a substance to flow or pour � Example Thick liquids like honey are very viscous And thin liquids like water are less viscous

Hardness �A measure of the resistance of a solid to being scratched. � Example Diamond is very hard and chalk is very soft

Malleability � The ability of a substance to be hammered into a thin sheet or moulded. � Examples Aluminum foil is malleable but glass will break if hammered

Ductility � The ability of a substance to be pulled into thin strands or wires � Example Copper is very ductile

Electrical Conductivity � The ability of a substance to let an electric current pass through it � Example Copper has high conductivity but rubber has no conductivity

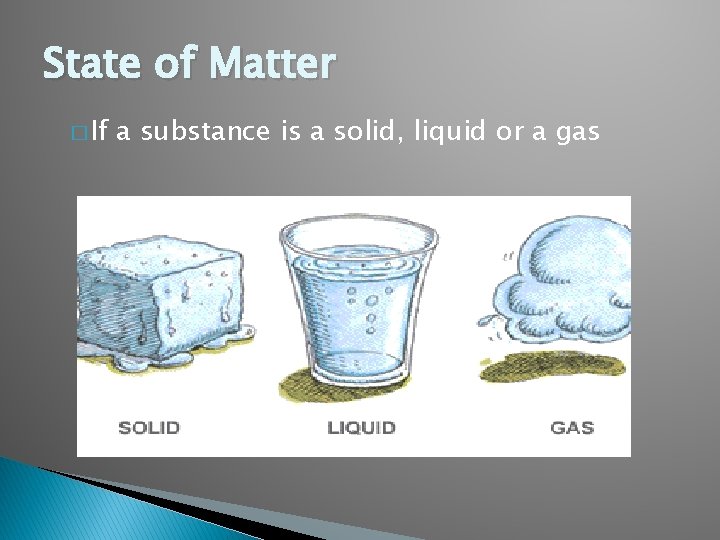
State of Matter � If a substance is a solid, liquid or a gas

Melting Point �The temperature a substance changes state from a solid to a liquid �Example Ice melts at 0 o. C

Boiling Point � The temperature a substance changes state from a liquid to a gas. � Example Water boils at 100 o. C

Freezing Point � The temperature a substance changes state from a liquid to a solid. � Example Water freezes at 0 o. C

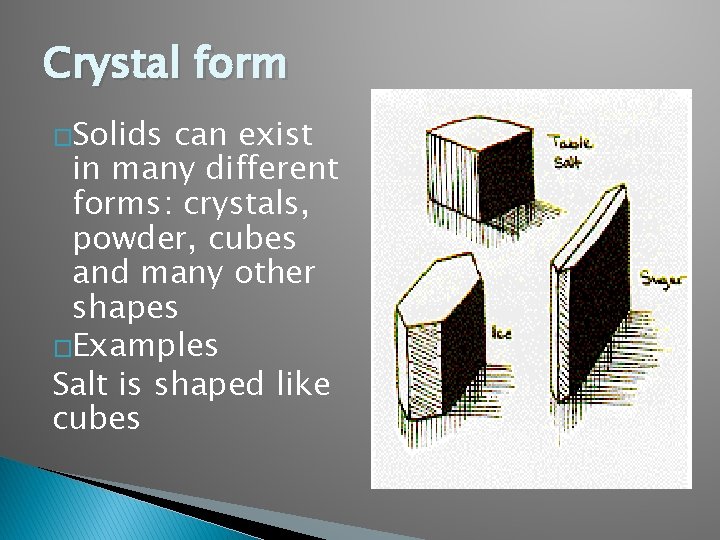
Crystal form �Solids can exist in many different forms: crystals, powder, cubes and many other shapes �Examples Salt is shaped like cubes

Solubility � The ability of a substance to dissolve in a solvent � Example Sugar is soluble in water but oil is not soluble in water

Density � The amount of matter per unit volume � Example Rock is more dense than water and foam

Other physical properties �Colour �Odour �Taste

Chemical Properties � Are properties that change the chemical nature of matter. There will be a chemical reaction. � The are properties that relate to how the substance changes in composition or how they interact with other substances.

Combustibility � The ability of a substance to burn � Example Gasoline is combustible but water is not

Reaction with Acid � The ability of a substance to react with an acid � Example Copper does not react with an acid but zinc reacts quickly with acid

Reaction with Water � The ability of a substance to react with a water � Example Lithium reacts violently when placed in water, copper does not react with water

Reaction With Oxygen � The ability of a substance to react with a oxygen gas � Example Iron rusts when it reacts with oxygen in air

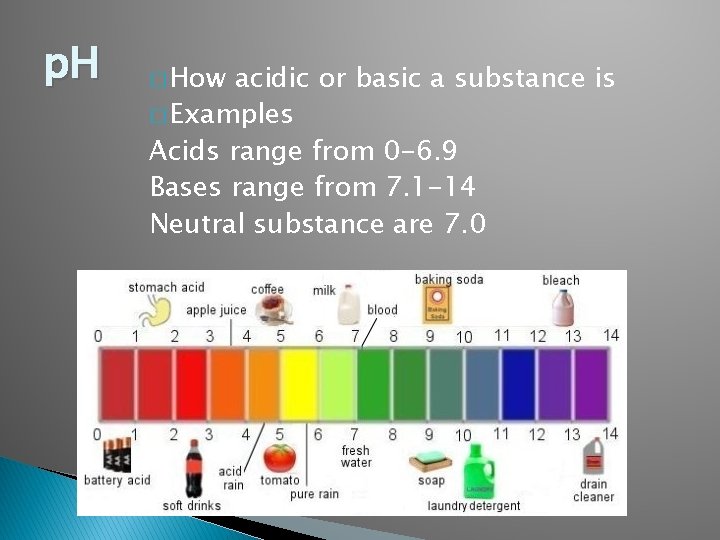
p. H � How acidic or basic a substance is � Examples Acids range from 0 -6. 9 Bases range from 7. 1 -14 Neutral substance are 7. 0

�HW: Practice Handout and page 182 #4, page 186 #2
 Antigentest åre
Antigentest åre Physical properties and chemical properties
Physical properties and chemical properties Properties of sulphuric acid
Properties of sulphuric acid A scientist performs an experiment, and an actor performs a
A scientist performs an experiment, and an actor performs a Chemical properties of dental materials
Chemical properties of dental materials Physical changes examples
Physical changes examples Physical and chemical properties of helium
Physical and chemical properties of helium Physical and chemical properties of boron
Physical and chemical properties of boron Physical and chemical properties interactive
Physical and chemical properties interactive Physical and chemical properties of silver
Physical and chemical properties of silver Properties of monosaccharides
Properties of monosaccharides Is dissolving salt in water physical or chemical
Is dissolving salt in water physical or chemical Water chemical and physical properties
Water chemical and physical properties Physical and chemical properties sorting activity
Physical and chemical properties sorting activity Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Physical change
Physical change Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Physical change and chemical change venn diagram
Physical change and chemical change venn diagram Physical change
Physical change Physical and chemical changes
Physical and chemical changes Physical and chemical phenomena
Physical and chemical phenomena