Physical v Chemical Physical Properties vs Chemical Properties








- Slides: 11

Physical v. Chemical

Physical Properties vs Chemical Properties ØPhysical Properties – can be observed without changing the identity of the substance Ø Examples: odor, color, taste, volume, state, hardness, density, melting and boiling points

Physical Properties vs Chemical Properties �Chemical Properties – its ability to form new substances Ø Examples: flammability, oxidation/corrosion

What is oxidation? ? � Oxidation is the interaction between oxygen molecules and all the different substances they may contact, from metal to living tissue. � Freshly cut apple turning brown � Bicycle fender rusting � Penny suddenly turning green *Prevent oxidation? Need a layer of protection � https: //www. youtube. com/watch? v=p 3 P 0 mjeb 5 UU

Physical or Chemical Properties? 1. 2. 3. 4. Boiling Point of an alcohol Diamonds are hard Sugars ferments to form alcohol A metal wire conducts an electric current

Physical Changes *Usually reversible (not always) �Physical Change – a change that does not affect the composition of a substance �Change like: � Crushing, �Most ripping, breaking or any phase change common physical (phase) changes: solid liquid gas *Chemical bonds are not broken

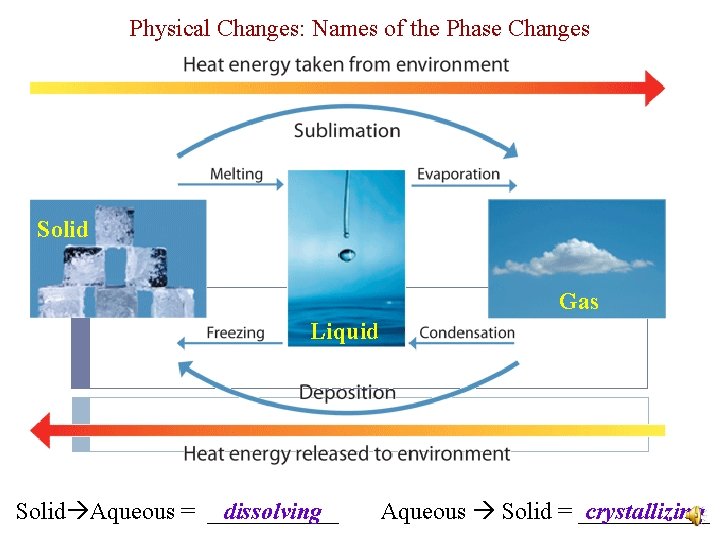
Physical Changes: Names of the Phase Changes Solid Gas Liquid Solid Aqueous = ______ dissolving Aqueous Solid = ______ crystallizing

Chemical Changes *Irreversible �Chemical Change – a change that a substance becomes a new, different substance. �Chemical changes are called reactions. �Changes like: � Burning, rusting, rotting or decomposing, fermenting, cooking, baking and other chemical reactions *Chemical bonds are broken and molecules rearrange to form new

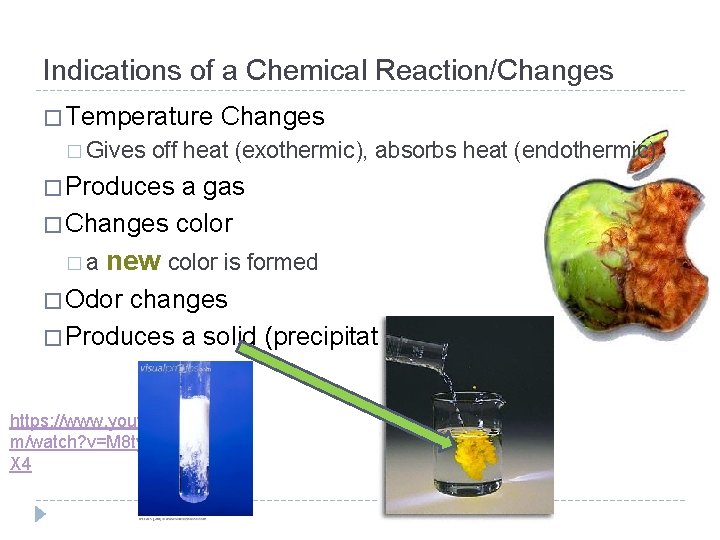
Indications of a Chemical Reaction/Changes � Temperature � Gives Changes off heat (exothermic), absorbs heat (endothermic) � Produces a gas � Changes color �a new color is formed � Odor changes � Produces a solid (precipitate) https: //www. youtube. co m/watch? v=M 8 tyjw. B 42 X 4

Physical or Chemical Changes? Ice melting to water 2. Burning Paper 3. Baking a cake 4. Putting blue food color in water 1.

Exit Slip Label as physical or chemical change: 1. Making ice cubes 2. Salt dissolving in water
 Chemical property definition
Chemical property definition Physical and chemical properties of sulphuric acid
Physical and chemical properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Physical properties of dental materials
Physical properties of dental materials Physical change
Physical change Chemical properties of helium
Chemical properties of helium Physical and chemical properties of boron
Physical and chemical properties of boron Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Physical and chemical properties interactive
Physical and chemical properties interactive Physical and chemical properties of silver
Physical and chemical properties of silver Properties of monosaccharides
Properties of monosaccharides Physical and chemical properties
Physical and chemical properties




















