Properties of Matter Physical Chemical Changes Physical Properties
















- Slides: 18

Properties of Matter Physical & Chemical Changes

Physical Properties n n Physical properties are observable Examples are: Mass n Volume n Density n Melting point n Boiling point n Hardness n Ability to conduct electricity n Physical State – solid, liquid, or gas n

Physical Properties Help ID Substances! n n A physical property can be used to identify a substance – shape, color, odor, texture Example: What is… Round n Orange n Smells rubbery n Bounces n A Basketball!! n

Magic Eggs Demo n n Observe what happens to the two eggs…. What physical property caused one to float and one to sink?

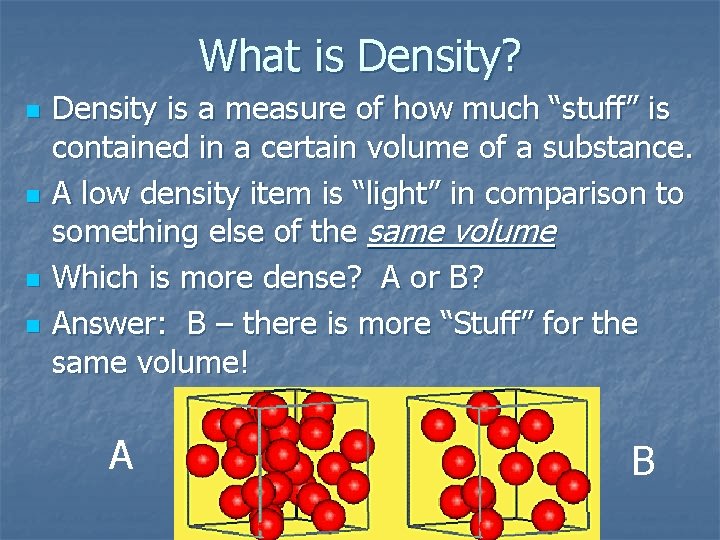
What is Density? n n Density is a measure of how much “stuff” is contained in a certain volume of a substance. A low density item is “light” in comparison to something else of the same volume Which is more dense? A or B? Answer: B – there is more “Stuff” for the same volume! A B

Online Lab n Go to the website, click on Online Density Lab and fill out the corresponding worksheet.

Density Equation D = m/V D = density M = mass V = volume Units! Mass must be in grams Volume may be in either cm 3 (if it is a solid) or m. L (if it is a liquid) Density = g/cm 3 or g/m. L

Example Problem #1 n n You are given a cube made out of an unknown substance. How would you determine its density? First you can measure one side – let’s say it is 2 cm. n Volume = l x w x h n n 2 x 2 = 8 cm 3 Then you can weigh it on a scale – let’s say its mass is 240 g D = m/V D = 240 g/8 cm 3 D = 30 g/cm 3

Example Problem #2 n n n You are given a cylinder made of an unknown substance. How would you determine its density? To find the volume you can drop it in a cylinder of water and note the change in volume – let’s say the water level rises 10 m. L Then you can weigh it on a scale – let’s say the mass is 100 g D = m/V D = 100 g/10 m. L D = 10 g/m. L

Float or Sink? Density determines whether an object will float or sink. n If an object is less dense than the fluid it is immersed in, it will float n If it is more dense, it will sink n Demos: n

Review n n Give four examples of a physical property. What is density? n n n Remember that density is a physical property and is unique to the matter – copper will have the same density, no matter how big or small the object is. How do you find volume if the object is a cube? A cylinder (round object)? Time to practice using the density equation….

Time for Labs!! n Cylinder Lab n Density Challenge #2

Chemical Properties Chemical properties are related to the elements that make up a substance. n They are not as easy to observe a physical properties n Examples: n n Flammability n Reactivity

Physical vs. Chemical Properties Physical properties are readily observable n Chemical properties can only be observed as the object is changing n Example – you do not know if an object is flammable until you set it on fire! n

Physical vs. Chemical Properties Physical Properties Chemical Properties Baking Soda White Powder Reacts with Vinegar Rubbing Alcohol Clear Liquid Flammable Red Food Coloring Red Color Reacts with Bleach to lose its Color Iron Malleable Reacts with Oxygen

Changes of Matter n n Physical Change n Affects one or more physical property, but does NOT change the identity of the substance n Break a piece of chalk – it is still chalk! Chemical Change n The substance changes into an entirely new substance with different properties n Not reversible (except by another chemical reaction) n Bake a cake – the cake is entirely different from the batter – new properties!

Physical or Chemical Change? Cutting Your Hair Physical Rusting Chemical Boiling Water Physical Dissolving Salt in Water Physical Burning Wood Chemical Frying an Egg Chemical

OK – one more Lab….