Physical and Chemical Properties Physical Properties Physical property







- Slides: 9

Physical and Chemical Properties

Physical Properties • Physical property- A characteristic that can be observed using one of the five senses • Physical Change- any change that can be reversed. • Common Physical properties. Color, state of matter, mass, weight, volume, size,

Physical Properties • Solubility- Amount of a substance that will dissolve in 100 grams of water • Melting point- The point at which a solid changes to a liquid • Boiling point- The point at which a liquid changes to a gas • Density- the amount of matter in a given object –D=M/V

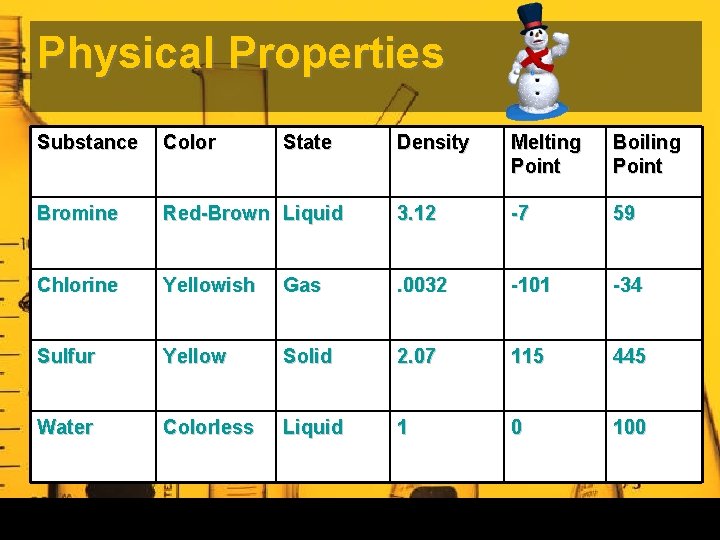
Physical Properties Substance Color Bromine State Density Melting Point Boiling Point Red-Brown Liquid 3. 12 -7 59 Chlorine Yellowish Gas . 0032 -101 -34 Sulfur Yellow Solid 2. 07 115 445 Water Colorless Liquid 1 0 100

Size Dependent Property • Size dependent property- a property that changes due to size ex. Mass, weight, length, volume

Size Independent Properties • Size independent properties- A property that does not change according to size ex. Density (mass/volume), Melting point, Boiling point.

Behavior • Behavior- the way a specific substance reacts – Example-Lodestone is magnetic

Chemical Properties • Chemical property- The ability of a substance to undergo change that alters its identity. • Chemical reaction- The change that alters a substances identity. ex. Burning, rusting

Chemical Changes • 1. 2. 3. Ways to Identify a chemical reaction Gas given off Change of color Change of temperature (chemical reactions usually create heat even if it is a very small amount)
 Is smell a physical property
Is smell a physical property Difference between chemical and physical change
Difference between chemical and physical change Physical property of blue color
Physical property of blue color Is brittleness a physical or chemical property
Is brittleness a physical or chemical property Is compressibility a physical or chemical property
Is compressibility a physical or chemical property Chemical properties of sulphuric acid
Chemical properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Thermal conductivity of dental materials
Thermal conductivity of dental materials Physical changes of matter
Physical changes of matter Hardness property of matter
Hardness property of matter