Chemistry Physical and Chemical Properties Physical Chemical Changes




















- Slides: 47

Chemistry Physical and Chemical Properties Physical & Chemical Changes © 2013 Michelle Brosseau, Mrs. Brosseau’s Binder

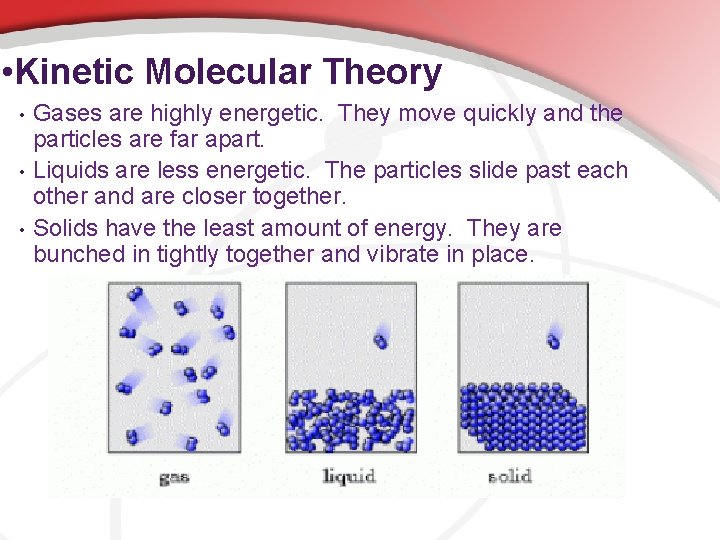
• Kinetic Molecular Theory • • • Gases are highly energetic. They move quickly and the particles are far apart. Liquids are less energetic. The particles slide past each other and are closer together. Solids have the least amount of energy. They are bunched in tightly together and vibrate in place.

• Classification of Matter • Pure substances is any single type of material. o Elements cannot be broken down any further. o Compounds are made up of elements.

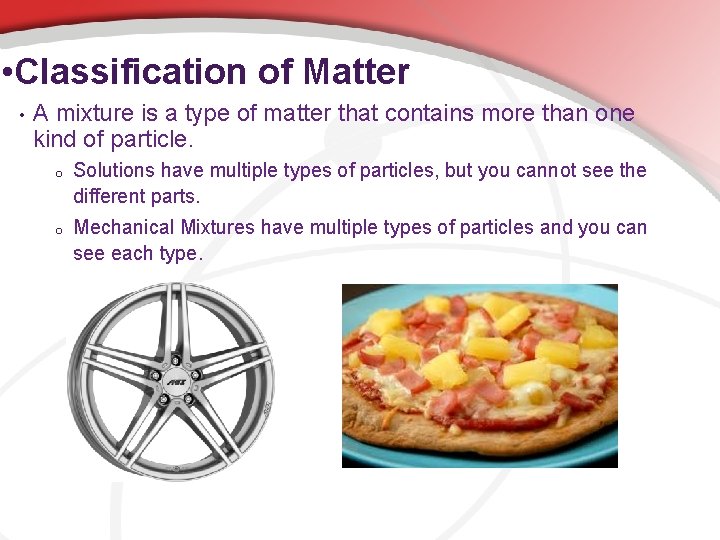
• Classification of Matter • A mixture is a type of matter that contains more than one kind of particle. o Solutions have multiple types of particles, but you cannot see the different parts. o Mechanical Mixtures have multiple types of particles and you can see each type.

• Bell Ringer 8/17/15 • Use your senses to describe this classroom.

• ESSENTIAL QUESTION • What properties can I use to describe matter?

• WHAT IS MATTER? • Matter is anything that has mass and takes up space. • 1. Matter is made up of tiny particles (Atoms & Molecules) • 2. Particles of Matter are in constant motion. • 3. Particles of Matter are held together by very strong electric forces • 4. There are empty spaces between the particles of matter that are very large compared to the particles themselves. • 5. Each substance has unique particles that are different from the particles of other substances.

• HOW DO I DESCRIBE MATTER? • 1) PHYSICAL PROPERTIES • WE USE OUR SENSES: -HEAR -SEE -TASTE (ONLY IF WE KNOW NOTHING IS POISONOUS) -FEEL (ONLY IF WE KNOW NOTHING IS EXTREMELY ACIDIC OR EXTREMELY BASIC) -SMELL (HOW DO WE CORRECTLY SMELL IN THE LAB? ) • WE USE MEASURING DEVICES: -MASS -TEMPERATURE -CALCULATE DENSITY • Physical -DIMENSIONS properties can be determined without destroying the substance.

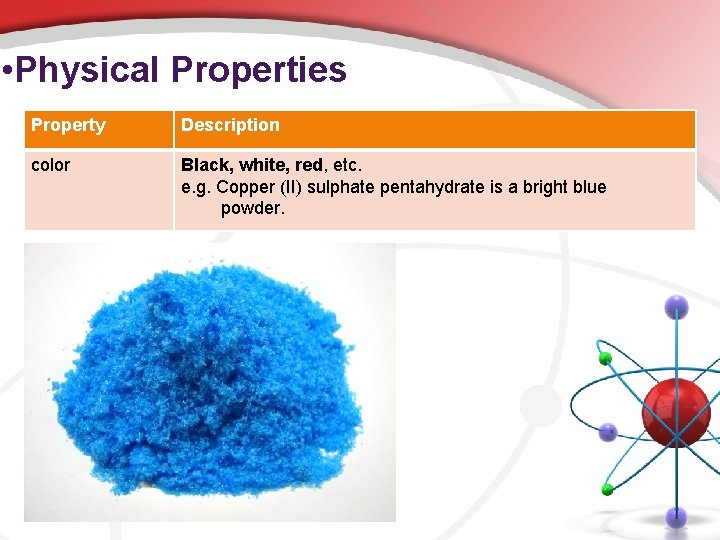
• Physical Properties Property Description color Black, white, red, etc. e. g. Copper (II) sulphate pentahydrate is a bright blue powder.

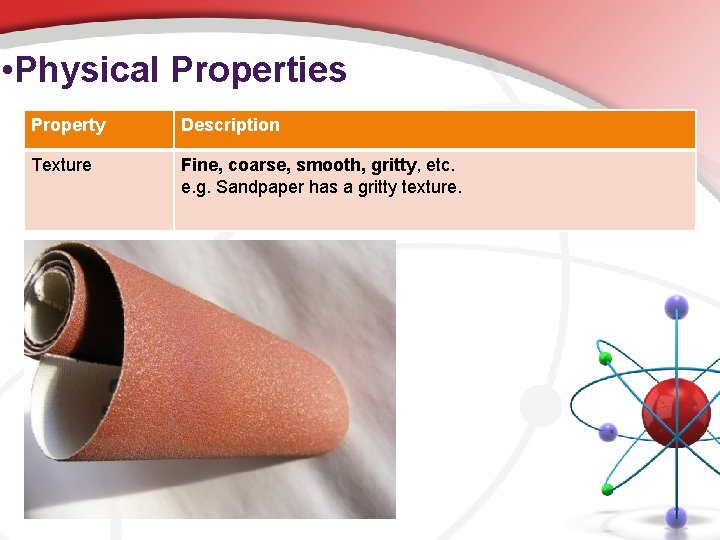
• Physical Properties Property Description Texture Fine, coarse, smooth, gritty, etc. e. g. Sandpaper has a gritty texture.

• Physical Properties Property Description Odorless, spicy, sharp, burnt, etc. e. g. Skunks emit an odour that can be described as a combination of rotten eggs, garlic, and burnt rubber.

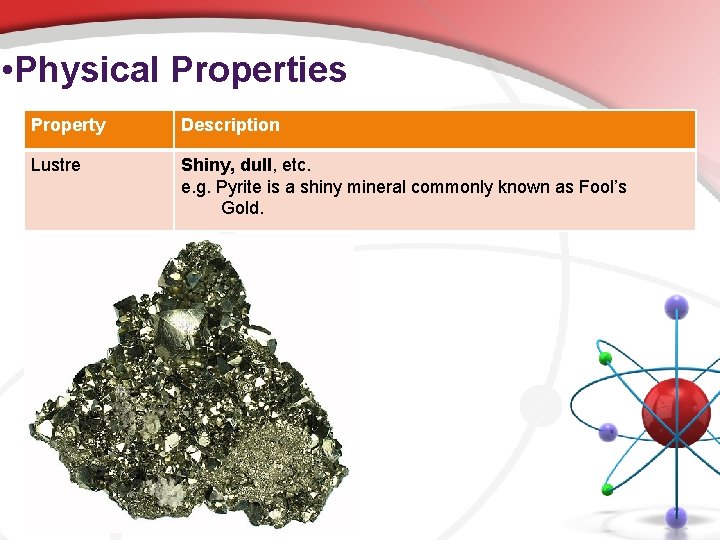
• Physical Properties Property Description Lustre Shiny, dull, etc. e. g. Pyrite is a shiny mineral commonly known as Fool’s Gold.

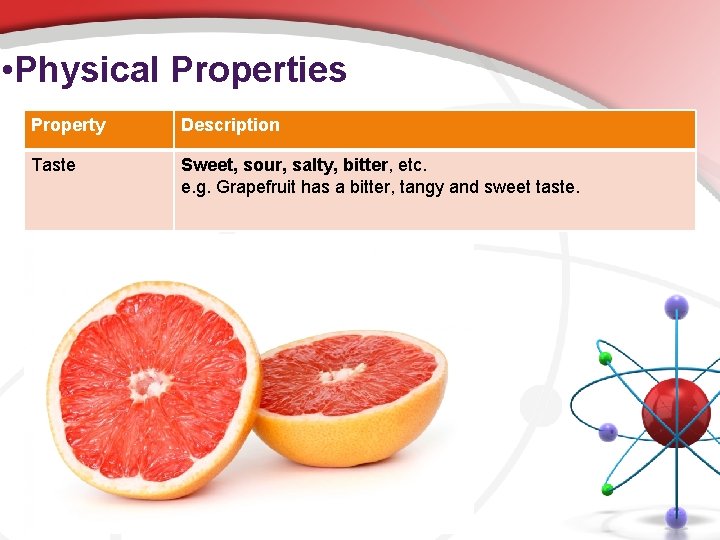
• Physical Properties Property Description Taste Sweet, sour, salty, bitter, etc. e. g. Grapefruit has a bitter, tangy and sweet taste.

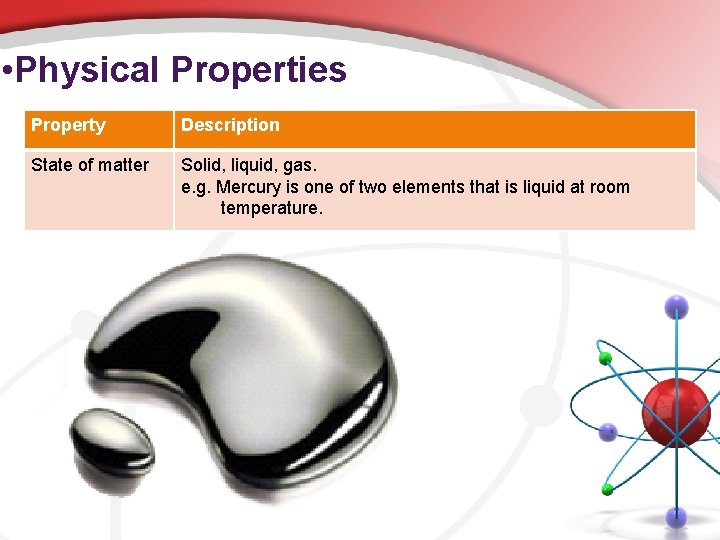
• Physical Properties Property Description State of matter Solid, liquid, gas. e. g. Mercury is one of two elements that is liquid at room temperature.

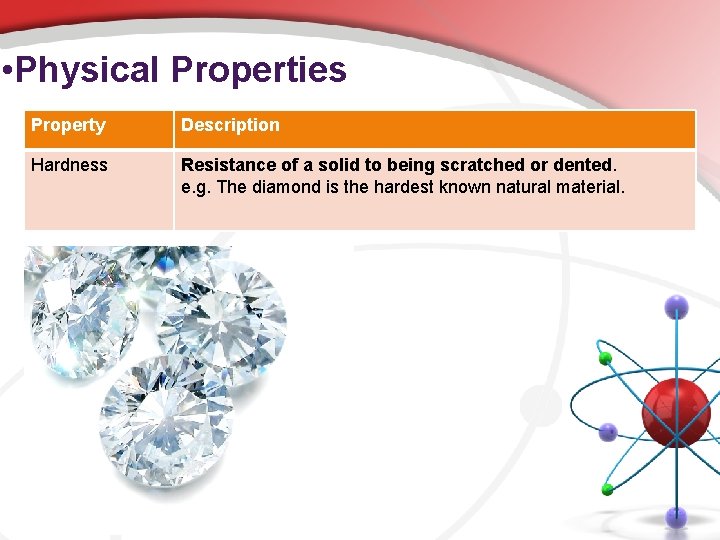
• Physical Properties Property Description Hardness Resistance of a solid to being scratched or dented. e. g. The diamond is the hardest known natural material.

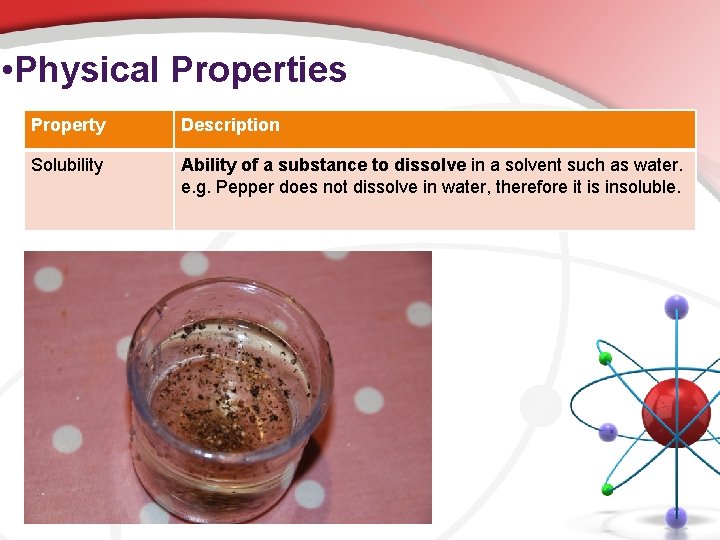
• Physical Properties Property Description Solubility Ability of a substance to dissolve in a solvent such as water. e. g. Pepper does not dissolve in water, therefore it is insoluble.

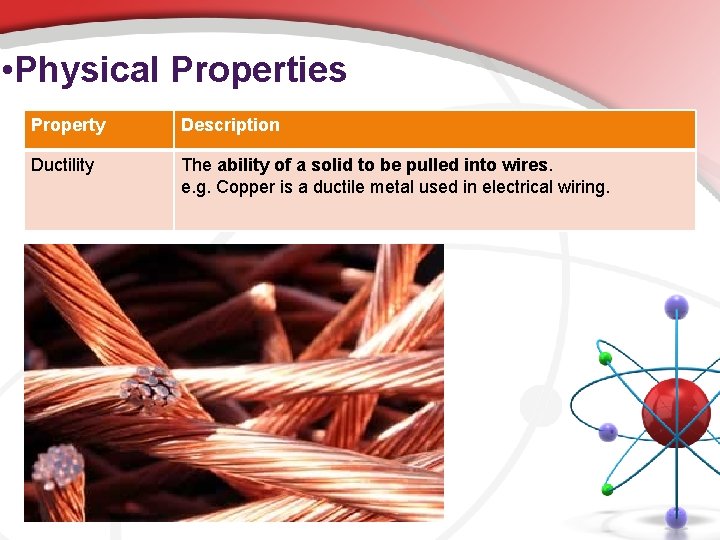
• Physical Properties Property Description Ductility The ability of a solid to be pulled into wires. e. g. Copper is a ductile metal used in electrical wiring.

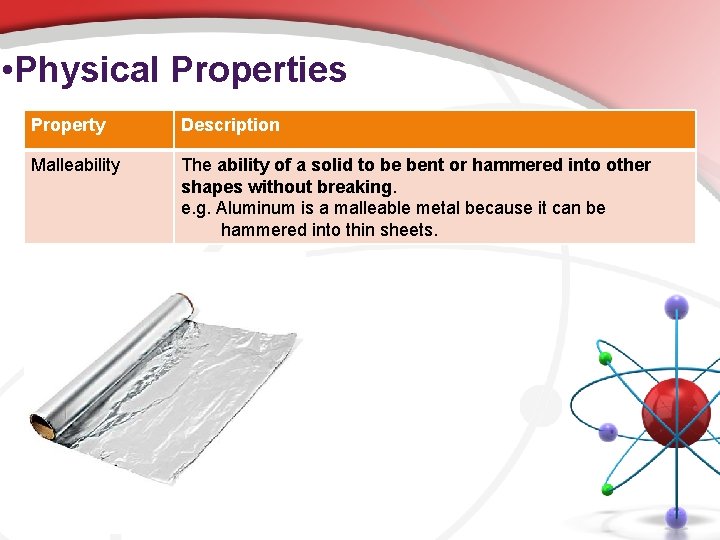
• Physical Properties Property Description Malleability The ability of a solid to be bent or hammered into other shapes without breaking. e. g. Aluminum is a malleable metal because it can be hammered into thin sheets.

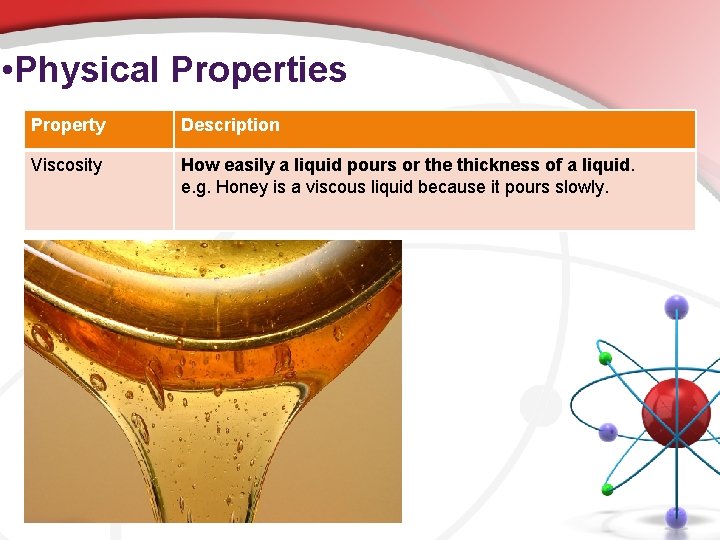
• Physical Properties Property Description Viscosity How easily a liquid pours or the thickness of a liquid. e. g. Honey is a viscous liquid because it pours slowly.

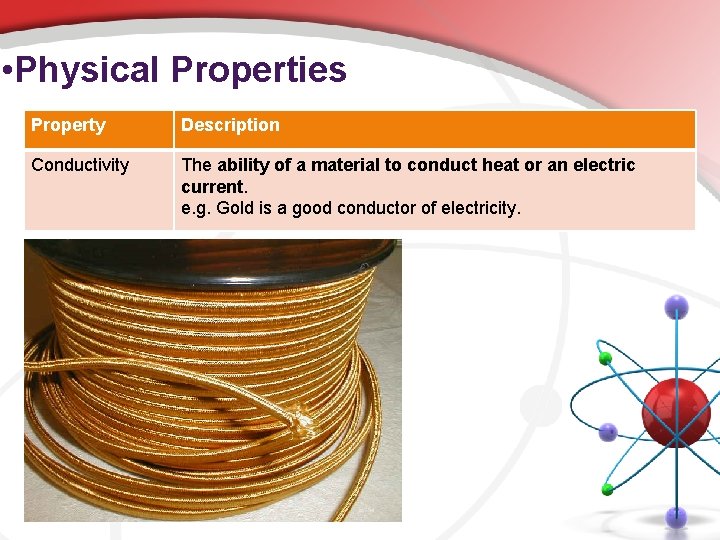
• Physical Properties Property Description Conductivity The ability of a material to conduct heat or an electric current. e. g. Gold is a good conductor of electricity.

• Physical Properties Property Description Mass The amount of matter in a substance. e. g. The heaviest dog in the world has a mass of 282 pounds.

• Physical Properties Property Description Temperature The measure of heat energy of a substance. e. g. The highest temperature ever recorded was 56. 7°C in Death Valley, California

• Physical Properties Property Description Dimensions The measure of the size of something in a particular direction, such as the length, width, height, or diameter. e. g. The longest finger nail measures 1. 3 meters.

• Physical Properties Property Description Density The relationship between the mass of the substance and how much space it takes up. e. g. Water is less dense than oil which is why oil appears to float on top of water. D=m/v

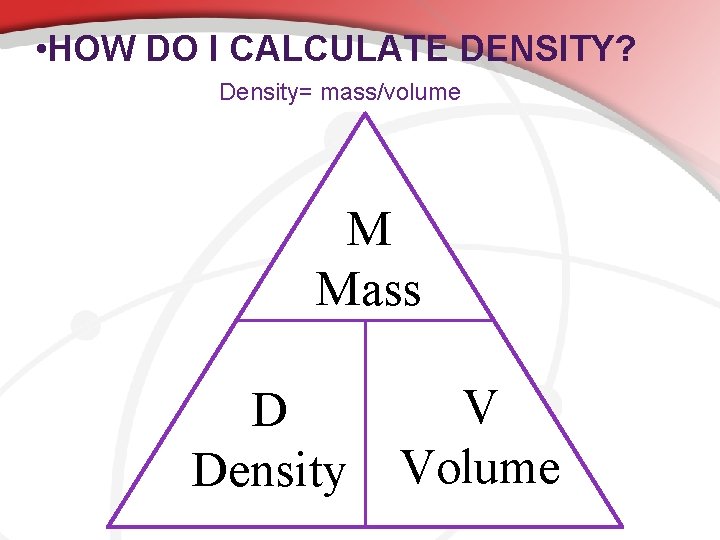
• HOW DO I CALCULATE DENSITY? Density= mass/volume M Mass D Density V Volume

• BASIC DENSITY PROBLEMS • CALCULATE THE DENSITY OF AN OBJECT WITH A MASS OF 30 g AND A VOLUME OF 15 cm 3 • CALCULATE THE DENSITY OF AN OBJECT WITH A MASS OF 28 g AND A VOLUME OF 7 cm 3 • WHAT IS THE MASS OF AN OBJECT WITH A DENSITY OF 4 g/cm 3 AND A VOLUME OF 3 cm 3

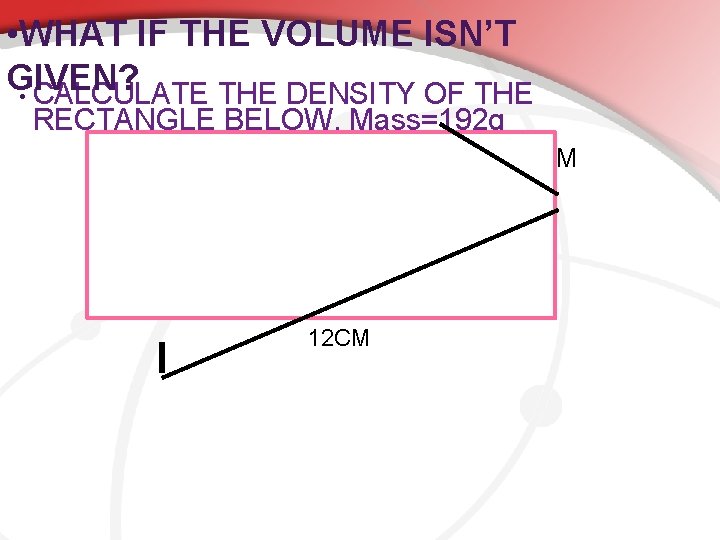
• WHAT IF THE VOLUME ISN’T GIVEN? • CALCULATE THE DENSITY OF THE RECTANGLE BELOW. Mass=192 g 8 CM 12 CM

• Bell Ringer 8/18/15 -4 th • Calculate the Density of an object with a mass of 16 g and 3 a volume of 8 cm. • List as many physical properties as you can.

• Bell Ringer 8/18/15 -5&6 • What are physical properties? • List as many physical properties as you can.

• Bell Ringer 8/18 Come in quietly. Please get out one sheet of notebook paper and a pencil. Place everything else on the floor. • 1. Calculate the density of the following object: Length: 5 cm Width: 2 cm Height: 2 cm Mass: 5 g - 2. Tell me everything you know about physical properties. (This includes examples!) -

• Chemical Properties A chemical property describes the behavior of a substance as it becomes a new substance -Flammability -Combustion -Corrosion - Tarnish - Rust - Reactivity - Oxidation - Chemical Property

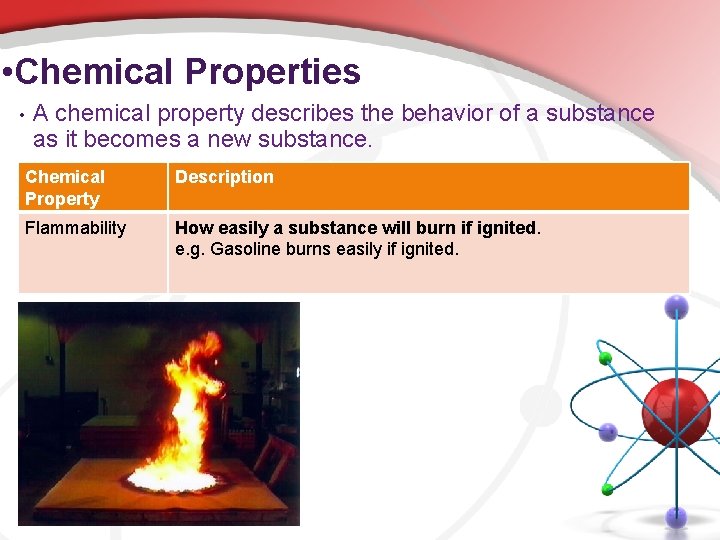
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Flammability How easily a substance will burn if ignited. e. g. Gasoline burns easily if ignited.

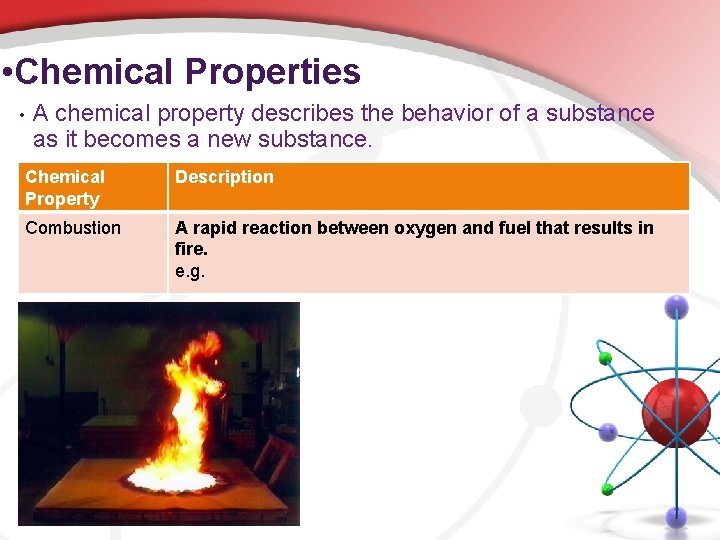
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Combustion A rapid reaction between oxygen and fuel that results in fire. e. g.

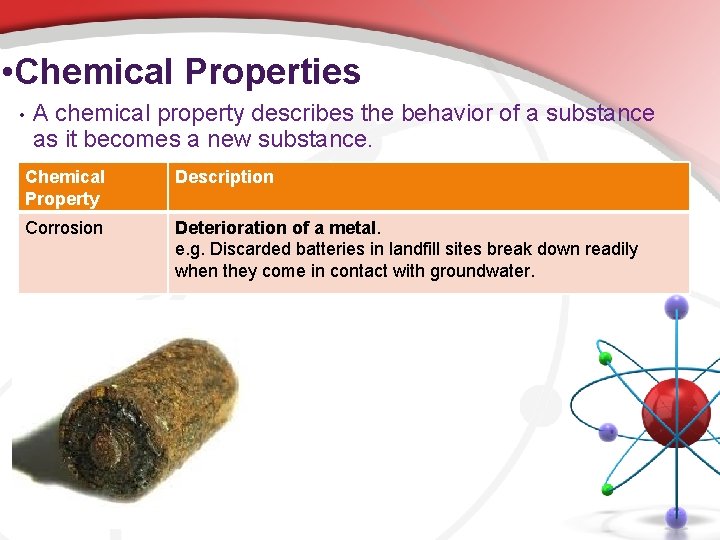
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Corrosion Deterioration of a metal. e. g. Discarded batteries in landfill sites break down readily when they come in contact with groundwater.

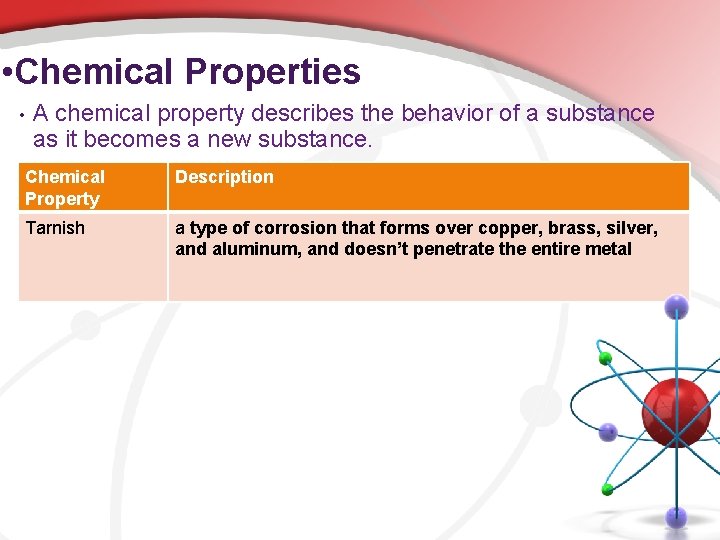
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Tarnish a type of corrosion that forms over copper, brass, silver, and aluminum, and doesn’t penetrate the entire metal

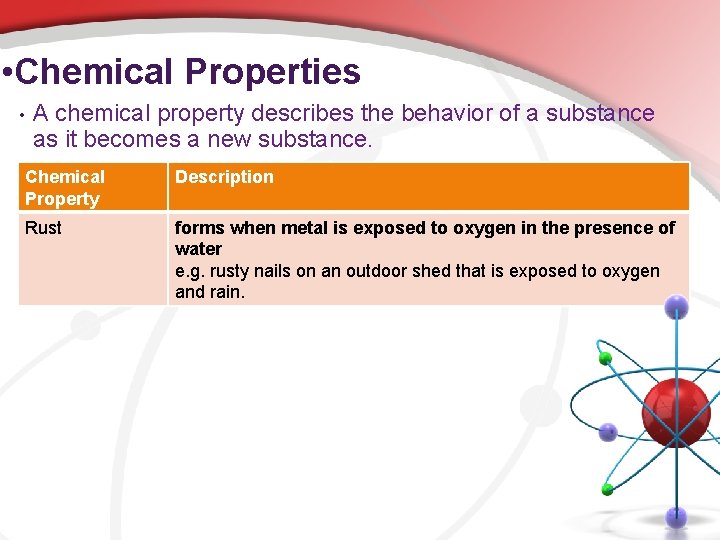
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Rust forms when metal is exposed to oxygen in the presence of water e. g. rusty nails on an outdoor shed that is exposed to oxygen and rain.

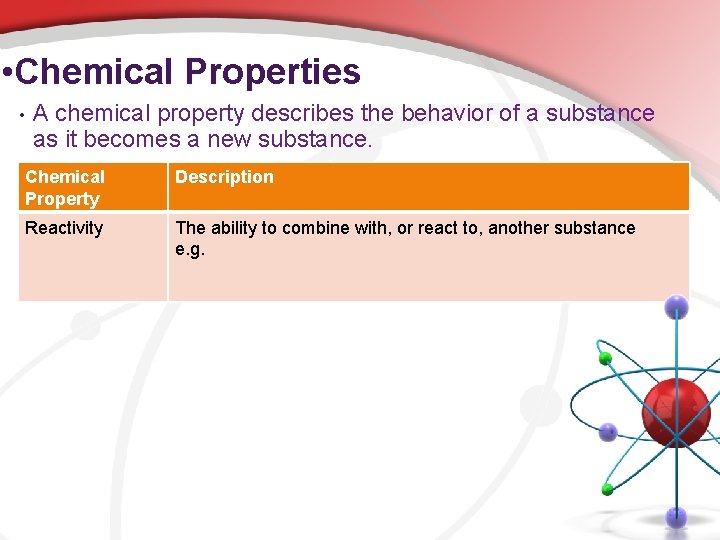
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Reactivity The ability to combine with, or react to, another substance e. g.

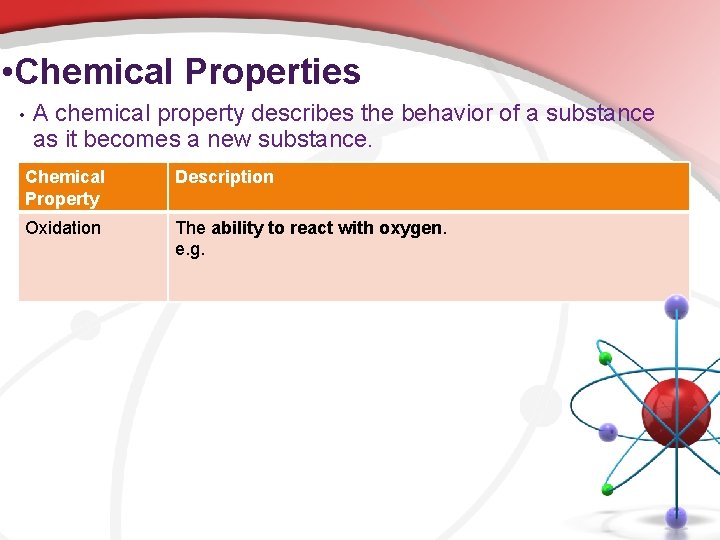
• Chemical Properties • A chemical property describes the behavior of a substance as it becomes a new substance. Chemical Property Description Oxidation The ability to react with oxygen. e. g.

• Exit Slip • Get out a sheet of notebook paper. 1. What is a chemical property? How do they differ from physical properties? 2. What is flammability? Where have you seen flammability in your own life?

• Physical and Chemical Changes • We experience physical and chemical changes everyday when we cook an egg, burn gasoline in the car, freeze water to make ice cubes or mix vinegar and oil to make salad dressing.

• Physical Change • • In a physical change, the substance involved remains the same. Most physical changes are easy to reverse. Physical Change Description Changes of State e. g. Melting, Freezing, Boiling

• Physical Change Description Dissolving solids e. g. dissolving salt (solute) into water (solvent), making Koolinto liquids Aid

• Chemical Change • • • In a chemical change, the original substance is changed into one or more new substances. The new substances have different properties from the original substance. Most chemical changes are difficult to reverse.

• Clues that a Chemical Change has occurred: • A new color appears.

• Clues that a Chemical Change has occurred: • Heat or light is produced or absorbed.

• Clues that a Chemical Change has occurred: • Bubbles of gas are formed.

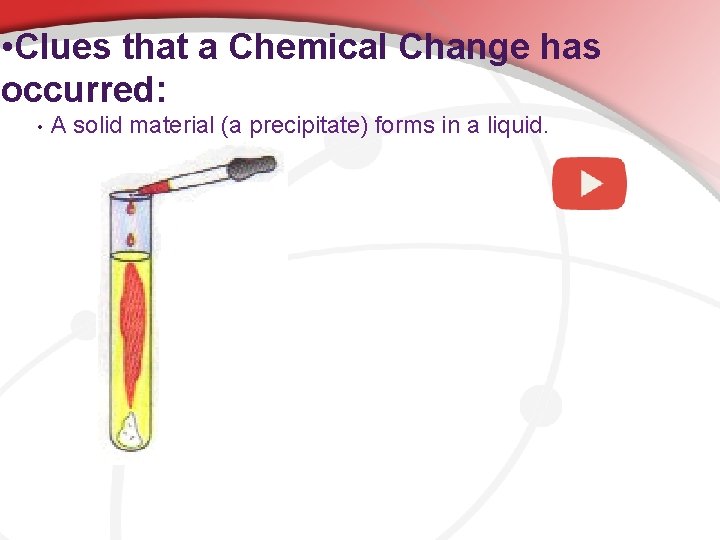
• Clues that a Chemical Change has occurred: • A solid material (a precipitate) forms in a liquid.
 Chemical changes example
Chemical changes example Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Chemical property of matter
Chemical property of matter Chemical properties and changes lesson 4
Chemical properties and changes lesson 4 Compare and contrast physical and chemical changes
Compare and contrast physical and chemical changes Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Physical and chemical changes
Physical and chemical changes Physical and chemical changes
Physical and chemical changes Sawing wood chemical or physical change
Sawing wood chemical or physical change Physical and chemical changes generation genius
Physical and chemical changes generation genius Is wadding paper a physical change
Is wadding paper a physical change What is a physical change
What is a physical change Differences between chemical and physical changes
Differences between chemical and physical changes Which is true about chemical change
Which is true about chemical change Whats the difference between physical and chemical changes
Whats the difference between physical and chemical changes Physical and chemical changes jeopardy
Physical and chemical changes jeopardy An example of a physical change
An example of a physical change Physical change example
Physical change example Lesson 3 physical and chemical changes answers
Lesson 3 physical and chemical changes answers Eating food physical or chemical change
Eating food physical or chemical change Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Section 1 chemical changes
Section 1 chemical changes Chemical.properties examples
Chemical.properties examples Changes in latitudes, changes in attitudes meaning
Changes in latitudes, changes in attitudes meaning Physical properties of sulphuric acid
Physical properties of sulphuric acid A scientist performs an experiment, and an actor performs a
A scientist performs an experiment, and an actor performs a Marginal percolation
Marginal percolation Physical changes of matter
Physical changes of matter Physical properties of helium
Physical properties of helium Physical properties of oxygen family
Physical properties of oxygen family Physical and chemical properties interactive
Physical and chemical properties interactive Physical properties of sodium
Physical properties of sodium Chemical properties of maltose
Chemical properties of maltose Physical properties
Physical properties Water chemical and physical properties
Water chemical and physical properties Physical and chemical properties sorting activity
Physical and chemical properties sorting activity Chemistry matter and its changes
Chemistry matter and its changes Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Which is a “big idea” for matter and change?
Which is a “big idea” for matter and change? Changes in wave properties sorting activity answer key
Changes in wave properties sorting activity answer key Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Combustion chemical reaction definition
Combustion chemical reaction definition Observing chemical change worksheet
Observing chemical change worksheet Extensive and intensive examples
Extensive and intensive examples Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Physical changes in old age
Physical changes in old age