Properties of Matter Types of Properties Changes Properties
































- Slides: 38

Properties of Matter Types of Properties & Changes

Properties of Matter (characteristics) A property is a characteristic that describes the substance.

In your Notebook…. . Create a T Chart like this…. . Physical Properties Chemical Properties

Under Physical Properties • A characteristic that can be OBSERVED without changing the COMPOSITION of the substance. Ex: density, ability to dissolve, break (make smaller) • If you can see it. • If you can measure it.

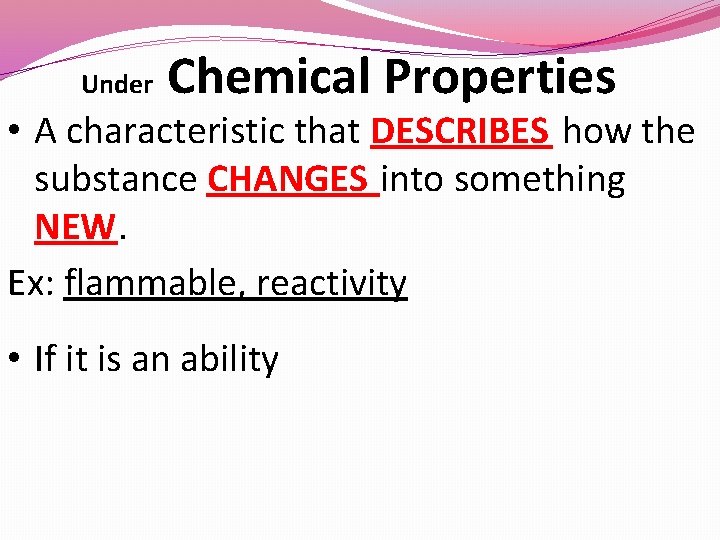
Under Chemical Properties • A characteristic that DESCRIBES how the substance CHANGES into something NEW. Ex: flammable, reactivity • If it is an ability

List the following as Examples Under Physical & Chemical Properties

Blue color

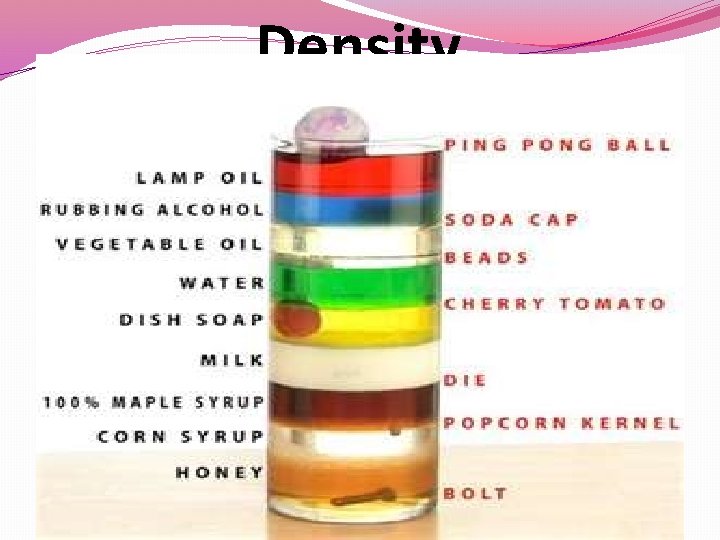
Density

Flammability

Solubility ability to dissolve

Reacts with acid to form H 2

SUPPORTS COMBUSTION

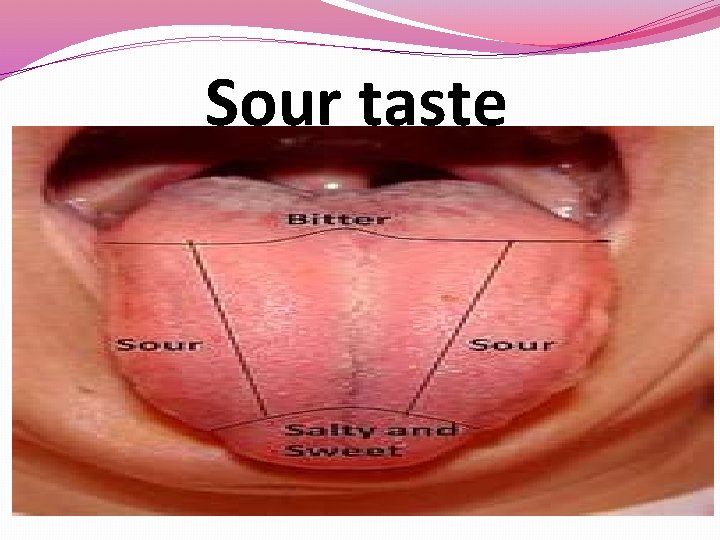
Sour taste

Melting point

Reacts with water to form a gas

Reacts with a base to form water

Hardness

Boiling point

Can neutralize a base

Luster

Odor

In your Notebook…. . Create a T Chart like this…. . Physical Changes Chemical Changes

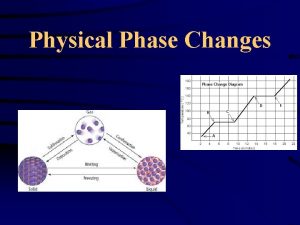
Under Physical Changes • A change that affects the form of a chemical substance but does not change the chemical composition of that substance. • Ex: Change in state (s, l, g) – Evaporation – Melting – dissolving

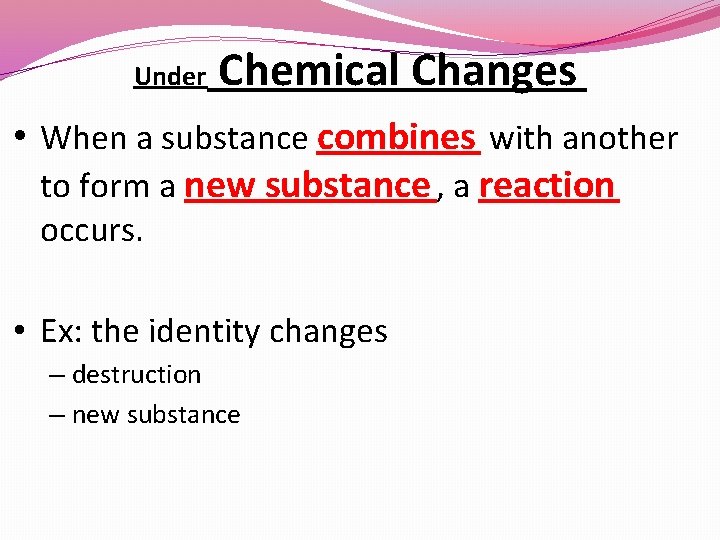
Under Chemical Changes • When a substance combines with another to form a new substance , a reaction occurs. • Ex: the identity changes – destruction – new substance

List the following as Examples Under Physical & Chemical Changes

Sodium hydroxide dissolves in water

HCl reacts with potassium hydroxide to produce a salt, water, and heat

A piece of wood is sliced in half

Water is heated and changed into steam

Water freezes and turns to ice

Condensation

Milk sours

Sugar dissolves in water

Wood rotting

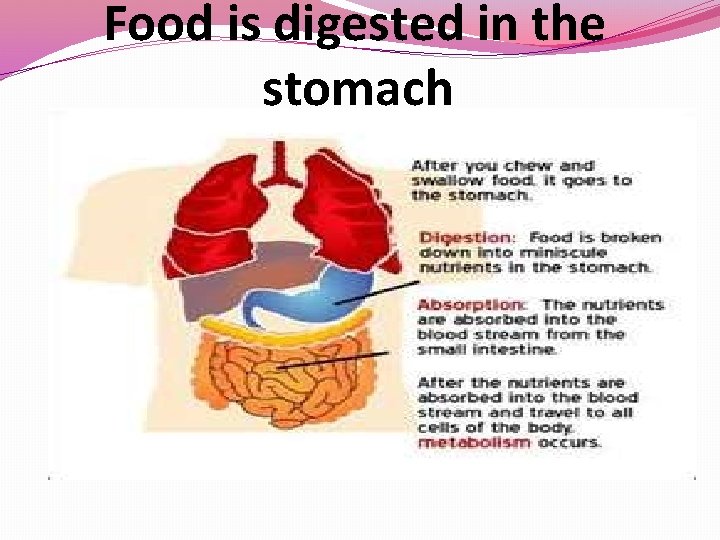
Food is digested in the stomach

Some other Properties

Intensive Properties DO NOT depend upon the amount of matter in the sample Ex: Density Color Hardness Malleability Melting Point

Extensive Properties depend upon the amount of matter in the sample Ex: Mass Volume Weight Temperature
 Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Big idea 9 changes in matter
Big idea 9 changes in matter Two types of changes
Two types of changes Elizabeth mulroney
Elizabeth mulroney Physical and chemical changes
Physical and chemical changes Physical properties
Physical properties 5 phases of matter
5 phases of matter Chemistry matter and its changes
Chemistry matter and its changes Change in state of matter
Change in state of matter Eating food physical or chemical change
Eating food physical or chemical change Stp law
Stp law Melting freezing evaporation condensation sublimation
Melting freezing evaporation condensation sublimation States of matter concept map
States of matter concept map Section 1 composition of matter
Section 1 composition of matter Gray and white matter
Gray and white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Gyrus and sulcus function
Gyrus and sulcus function Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Pallium telencephalon
Pallium telencephalon Ecological succession
Ecological succession Changes in wave properties sorting activity
Changes in wave properties sorting activity Chemical properties and changes lesson 4
Chemical properties and changes lesson 4 Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Types of software changes
Types of software changes E type software
E type software Adaptive maintenance in software engineering
Adaptive maintenance in software engineering State the properties of liquid state
State the properties of liquid state Properties of matter vocabulary
Properties of matter vocabulary Concept map properties of matter
Concept map properties of matter Objectives of properties of matter
Objectives of properties of matter Common properties of matter
Common properties of matter Classification and properties of matter
Classification and properties of matter Measurable properties of matter
Measurable properties of matter States of matter jeopardy
States of matter jeopardy Matter jeopardy
Matter jeopardy Properties of matter volume
Properties of matter volume