Changing States of Matter Concept Map 5 States




- Slides: 16

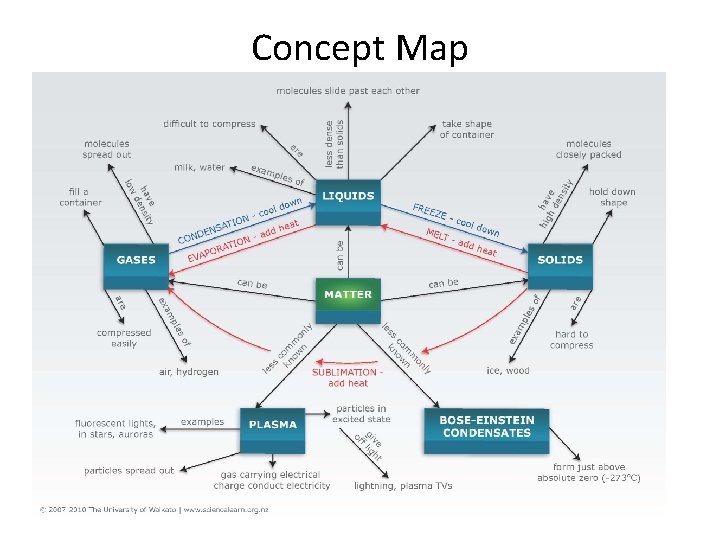
Changing States of Matter

Concept Map

5 States of Matter 3 Most Common States of Matter on Earth § Solids: Definite Shape and volume § Liquids: No definite shape but have a definite volume § Gases: No definite shape or volume. Uncommon States of Matter on Earth § Plasma § Bose-Einstein Condensates

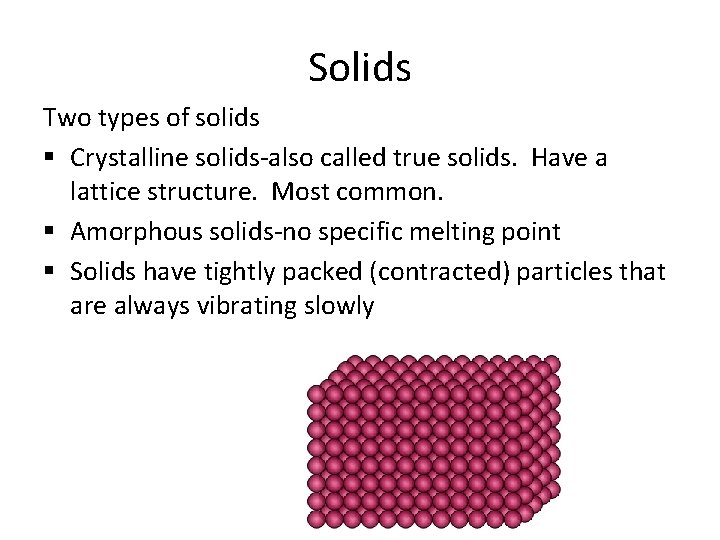
Solids Two types of solids § Crystalline solids-also called true solids. Have a lattice structure. Most common. § Amorphous solids-no specific melting point § Solids have tightly packed (contracted) particles that are always vibrating slowly

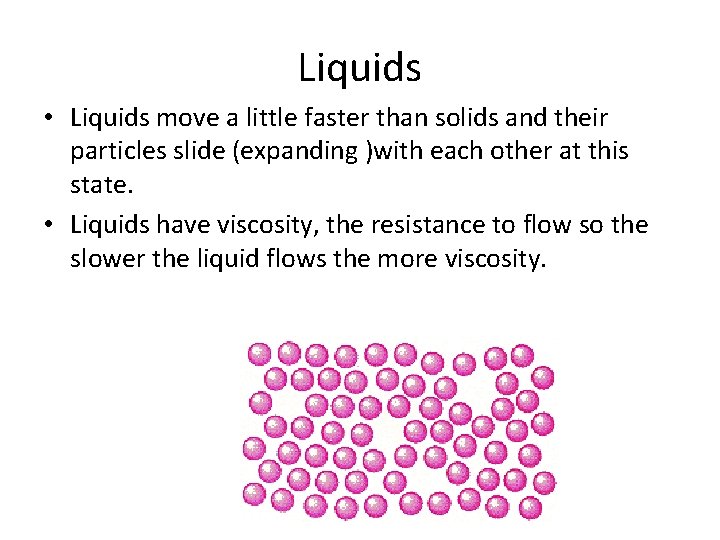
Liquids • Liquids move a little faster than solids and their particles slide (expanding )with each other at this state. • Liquids have viscosity, the resistance to flow so the slower the liquid flows the more viscosity.

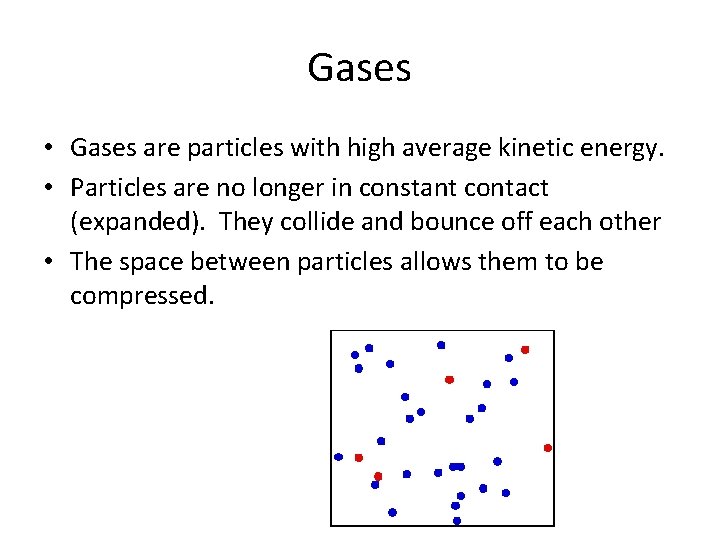
Gases • Gases are particles with high average kinetic energy. • Particles are no longer in constant contact (expanded). They collide and bounce off each other • The space between particles allows them to be compressed.

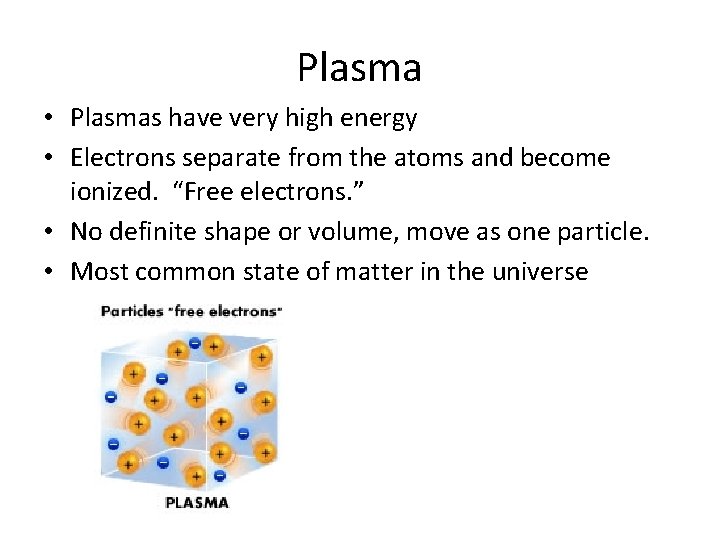
Plasma • Plasmas have very high energy • Electrons separate from the atoms and become ionized. “Free electrons. ” • No definite shape or volume, move as one particle. • Most common state of matter in the universe

Bose-Einstein Condensates • Just a fraction above absolute zero (0 Kelvin) and only for some elements – a BEC occurs. • The atoms start behaving like little waves and start overlapping one another until they eventually act like one wave and essentially become a superatom. • They are not bonded or mixed – they have become indistinguishable from one another, having the same qualities and existing in the same place. Bose-Einstein Condensates

Heat and Temperature • Heat is the amount of thermal energy. • Latent heat is the amount of heat needed to change from one state of matter to another. • Heat is measured in Joules • Temperature is the average amount of kinetic energy of the particles in a substance • Temperature is measured in Celsius or Kelvin

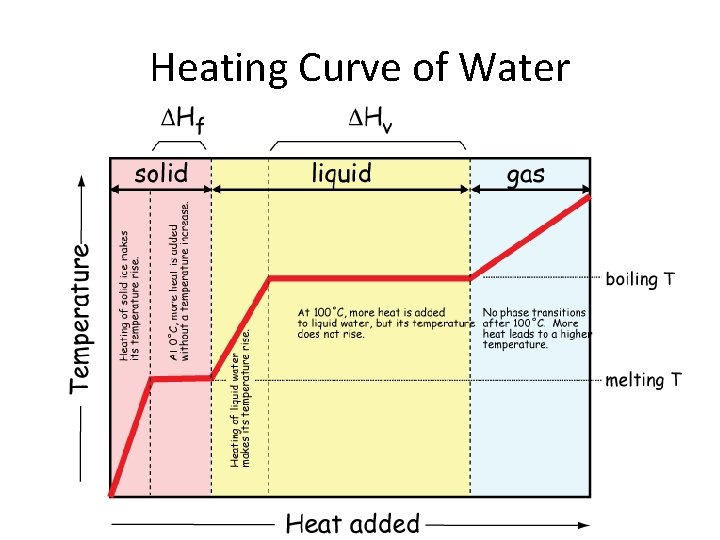
Heating Curve of Water

Changes in Heat • Latent Heat of Fusion is the amount of heat needed to break the bonds of a solid and turn it to a liquid. • Latent Heat of Vaporization is the amount of heat needed to move a liquid to a gas

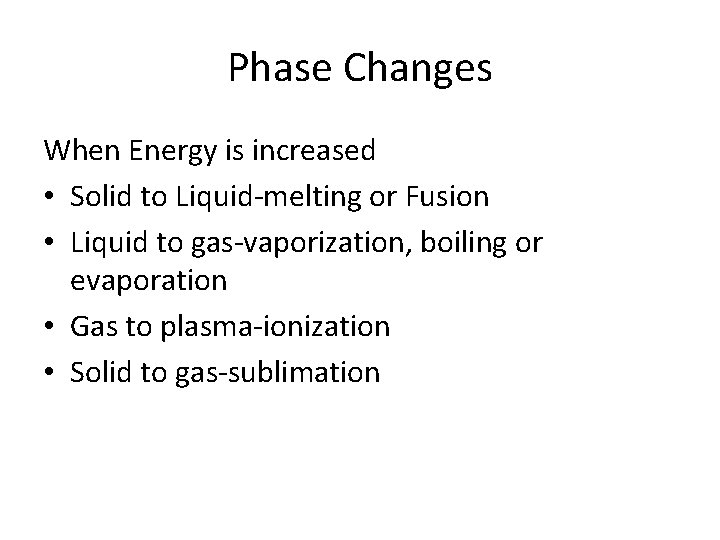
Phase Changes When Energy is increased • Solid to Liquid-melting or Fusion • Liquid to gas-vaporization, boiling or evaporation • Gas to plasma-ionization • Solid to gas-sublimation

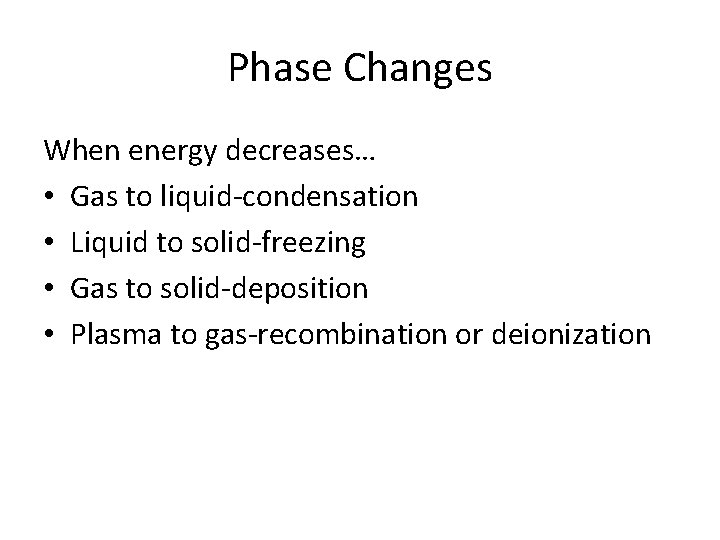
Phase Changes When energy decreases… • Gas to liquid-condensation • Liquid to solid-freezing • Gas to solid-deposition • Plasma to gas-recombination or deionization

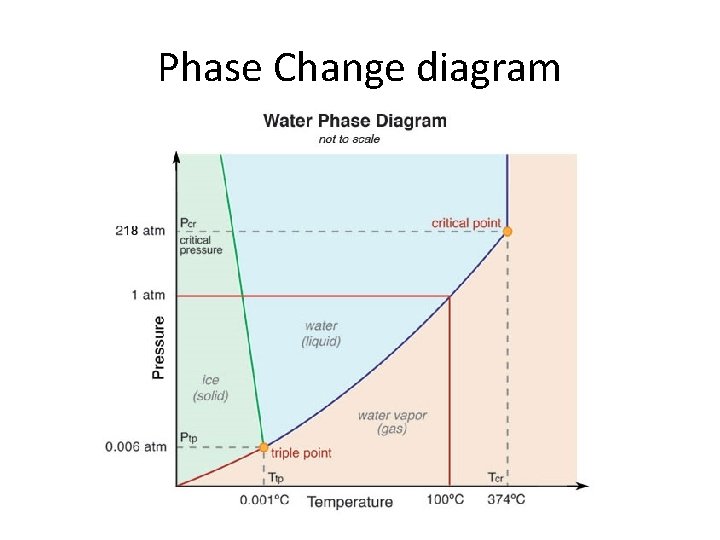
Phase Change diagram

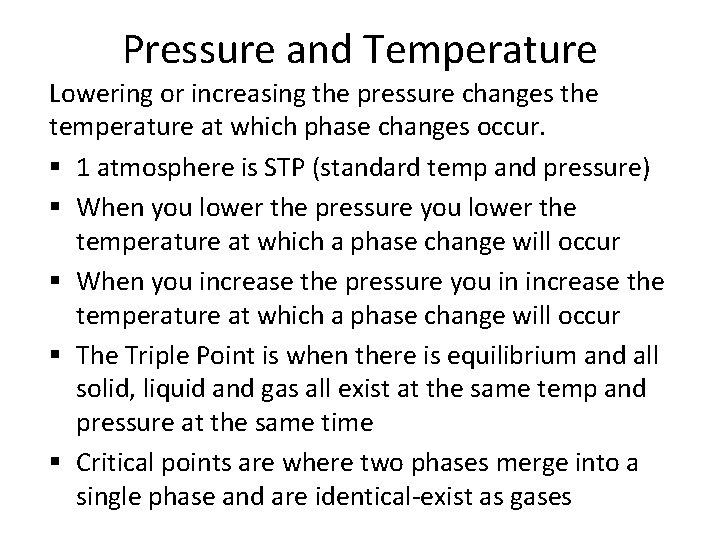
Pressure and Temperature Lowering or increasing the pressure changes the temperature at which phase changes occur. § 1 atmosphere is STP (standard temp and pressure) § When you lower the pressure you lower the temperature at which a phase change will occur § When you increase the pressure you in increase the temperature at which a phase change will occur § The Triple Point is when there is equilibrium and all solid, liquid and gas all exist at the same temp and pressure at the same time § Critical points are where two phases merge into a single phase and are identical-exist as gases

 Solid liquid gas concept map
Solid liquid gas concept map Changes in matter concept map
Changes in matter concept map Changing state
Changing state Physical change concept map
Physical change concept map Section 1 composition of matter
Section 1 composition of matter Grey matter in nervous system
Grey matter in nervous system Section 1 composition of matter
Section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Gyrus and sulcus function
Gyrus and sulcus function Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Matter
Matter Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Basic concepts of matter
Basic concepts of matter Phases of matter foldable
Phases of matter foldable Four phases of matter
Four phases of matter Four states of matter
Four states of matter






























