Changing States of Matter States of Matter There





















- Slides: 46






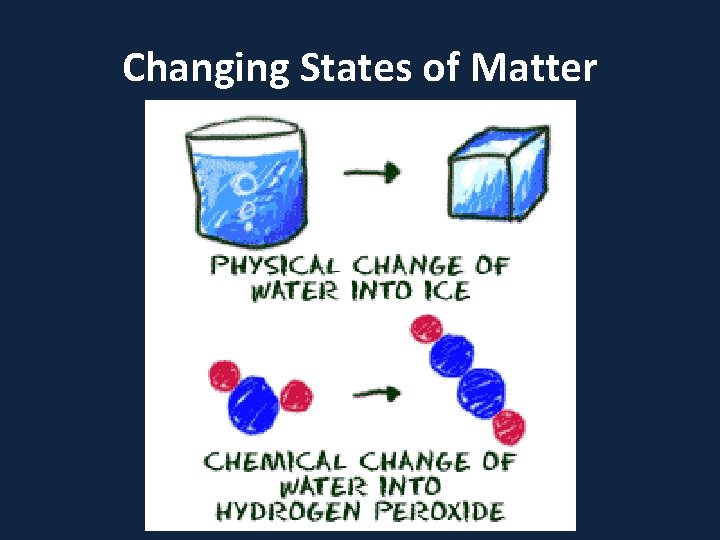
Changing States of Matter


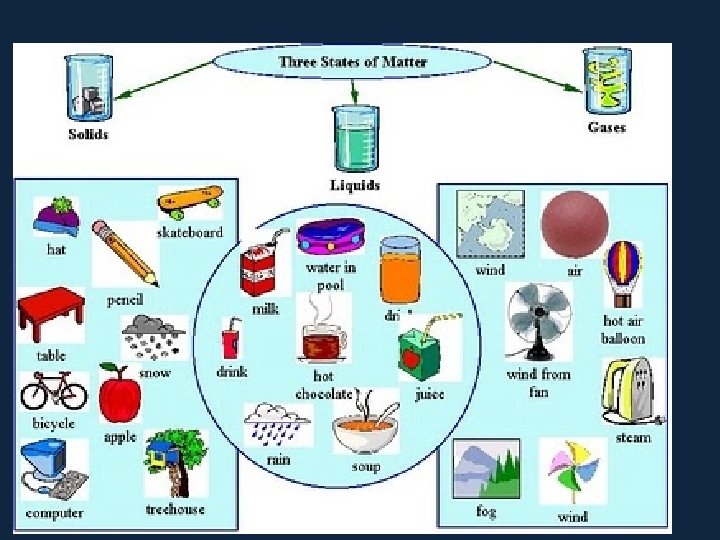
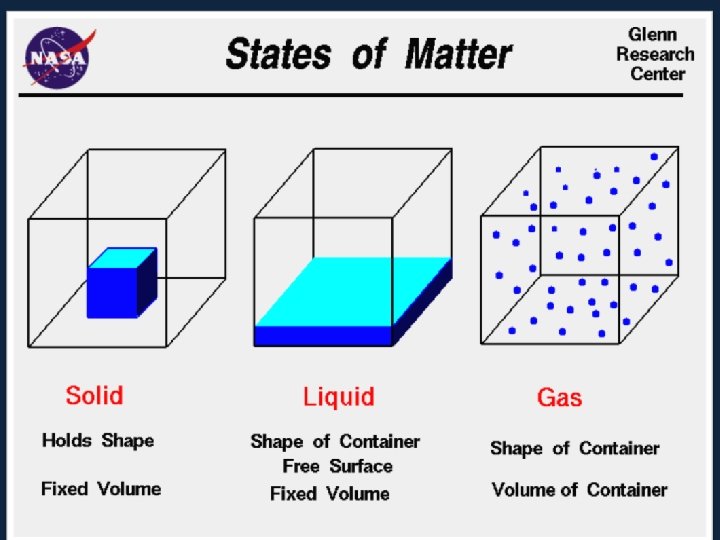
States of Matter. . . • There are 3 states of matter. SOLIDS, LIQUIDS and GASES. • Elements can exist in each. . . though at room temperature they prefer to be in one or the other. . . they have to be encouraged to change state. . . How? By adding ENERGY! • Water (H 2 O) exists as a solid, (ice), liquid (water) and a gas (steam).

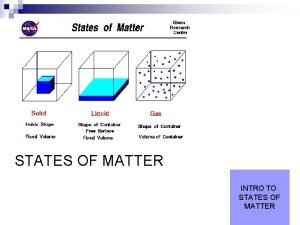
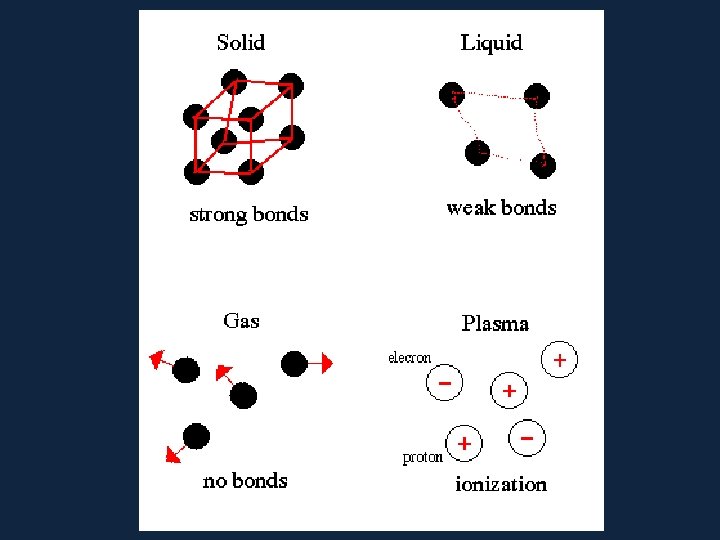
• In each state the atoms are arranged differently In SOLIDS –the atoms are held tightly in place by strong glue-like bonds. –At the most all they can do is vibrate. ������


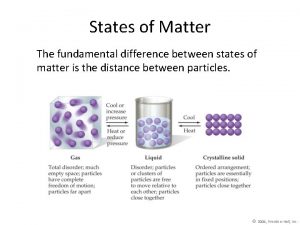
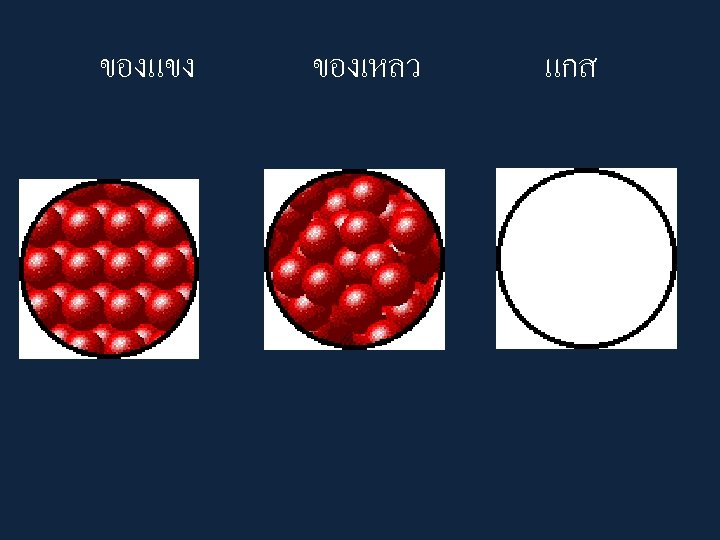
• Particles in a: – gas are well separated with no regular arrangement. – liquid are close together with no regular arrangement. – solid are tightly packed, usually in a regular pattern. • Particles in a: – gas vibrate and move freely at high speeds. – liquid vibrate, move about, and slide past each other. – solid vibrate (jiggle) but generally do not move from place to place.

In LIQUIDS –the forces that hold the atoms together are not as strong. –They can move over one another. –Liquids can change shape because of this.

In GASES –the atoms are free to move where they want. –they have lots of energy and can fill any space quickly.

Some Characteristics of Gases, Liquids and Solids and the Microscopic Explanation for the Behavior gas liquid assumes the shape and assumes the shape of the volume of its container part of the container which particles can move past one it occupies another particles can move/slide past one another compressible not easily compressible lots of free space between little free space between particles solid retains a fixed volume and shape rigid - particles locked into place flows easily particles can move past one particles can move/slide another past one another does not flow easily rigid - particles cannot move/slide past one another not easily compressible little free space between particles



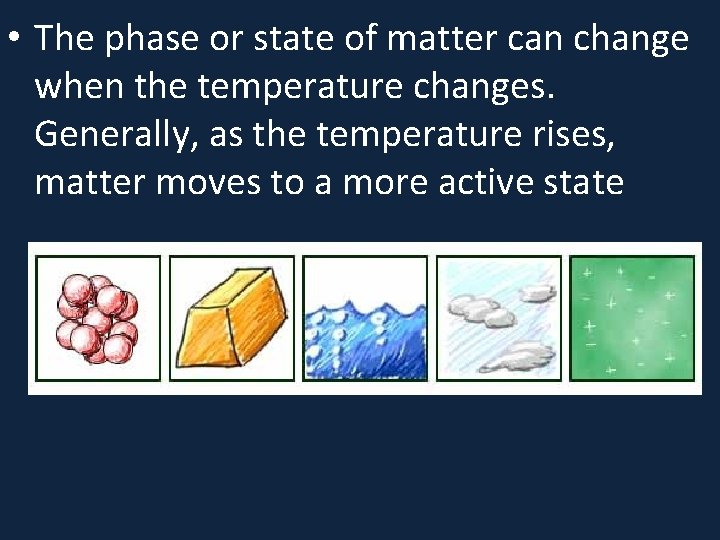
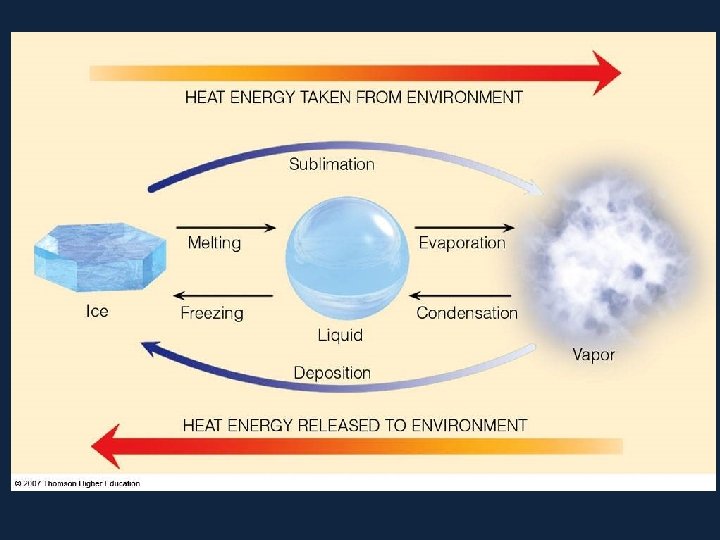
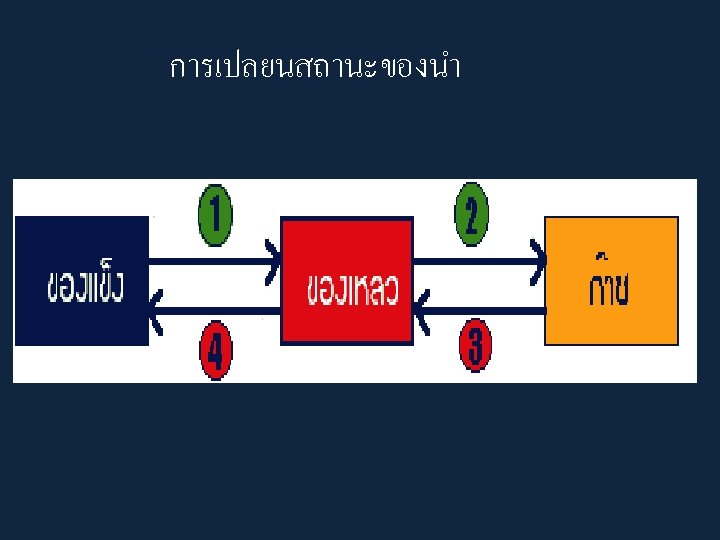
• The phase or state of matter can change when the temperature changes. Generally, as the temperature rises, matter moves to a more active state

• Phase describes a physical state of matter. The key word to notice is physical. Things only move from one phase to another by physical means. If energy is added (like increasing the temperature or increasing pressure) or if energy is taken away (like freezing something or decreasing pressure) you have created a physical change.

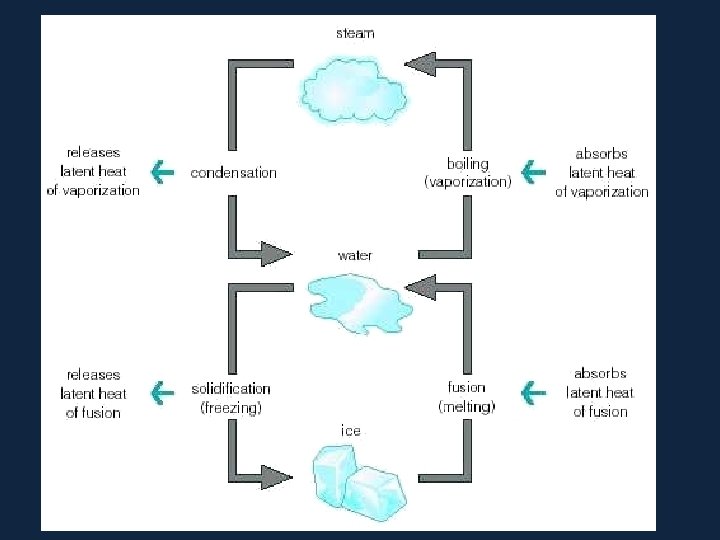
• One compound or element can move from phase to phase, but still be the same substance. • You can see water vapor over a boiling pot of water. That vapor (or gas) can condense and become a drop of water

• Solids have the lowest amount of energy • Liquids have the second least amount of energy • Gases are the second most energetic of the four • And Plasma has the most amount of energy of them all

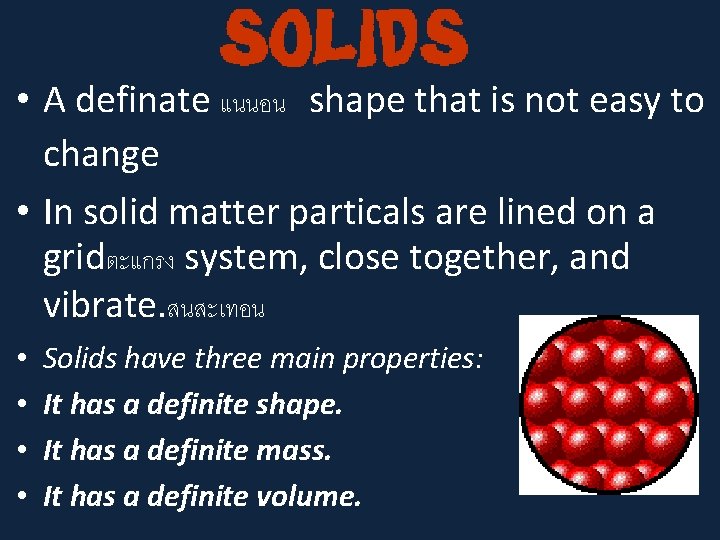
• A definate แนนอน shape that is not easy to change • In solid matter particals are lined on a gridตะแกรง system, close together, and vibrate. สนสะเทอน • • Solids have three main properties: It has a definite shape. It has a definite mass. It has a definite volume.

• Substance that is neither solid or gas and flows like water. • In solid matter particals are lined on a grid system, close together, and vibrate. • • Liquids have three main properties: It does not have a definite shape. It has a definite mass. It has a definite volume.

• A substance that has no shape, or size and can expand���� without limits • The molecules move very easily and in random directions • Gases have three main properties: • It does not have a definite shape. • It does not have a definite mass. • It does not have a definite volume.

Plasma • A highly ionized gas consisting of an almost equal numer of positive ion and free electrons • Plasma is arranged ���� by atoms called ions and electrons.

• The ice in this picture is a solid. The water in the picture is a liquid. In the air there is water vapor, which is a gas.


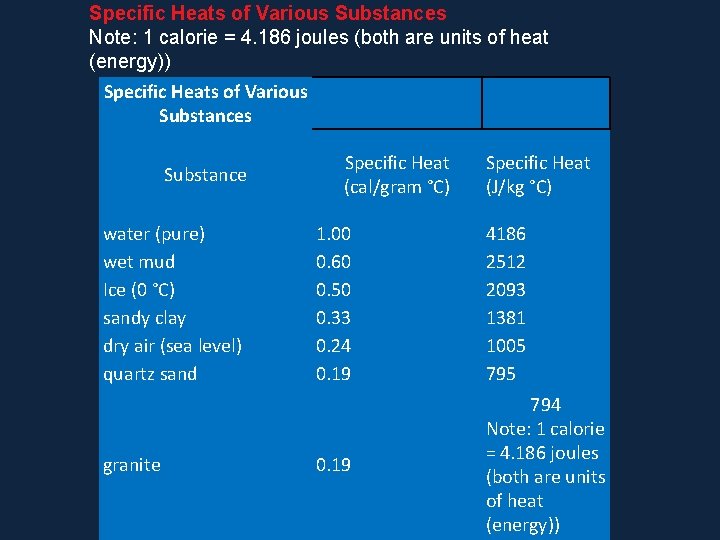
Specific Heats of Various Substances Note: 1 calorie = 4. 186 joules (both are units of heat (energy)) Specific Heats of Various Substance water (pure) wet mud Ice (0 °C) sandy clay dry air (sea level) quartz sand granite Specific Heat (cal/gram °C) Specific Heat (J/kg °C) 1. 00 0. 60 0. 50 0. 33 0. 24 0. 19 4186 2512 2093 1381 1005 795 0. 19 794 Note: 1 calorie = 4. 186 joules (both are units of heat (energy))


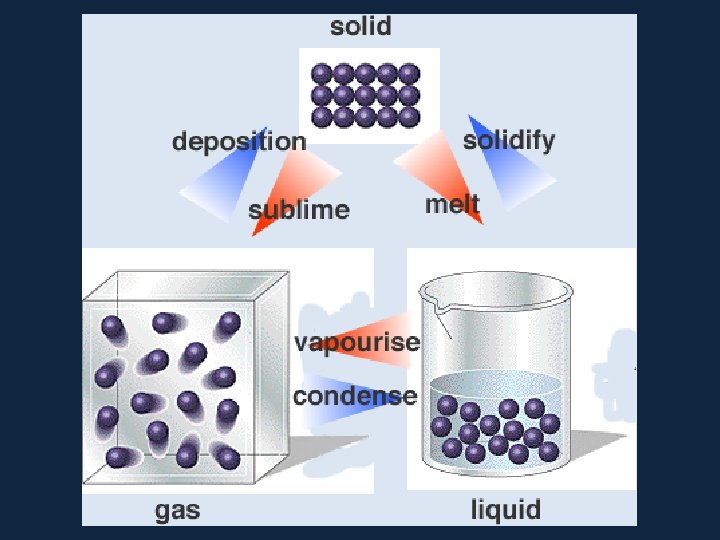
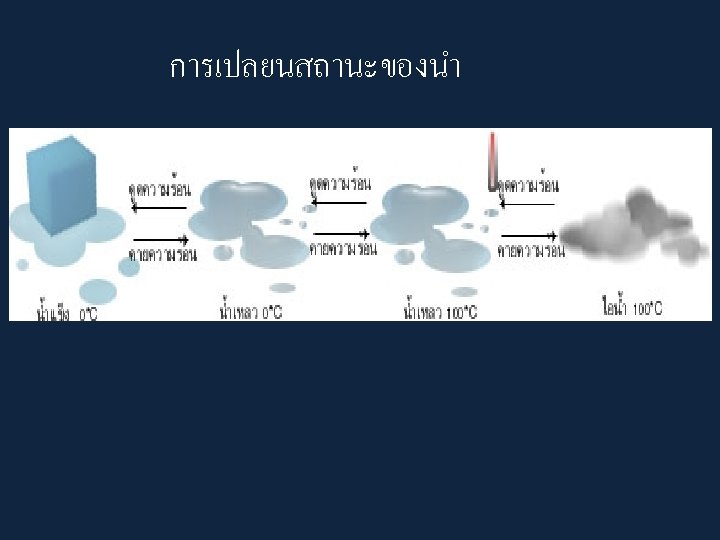
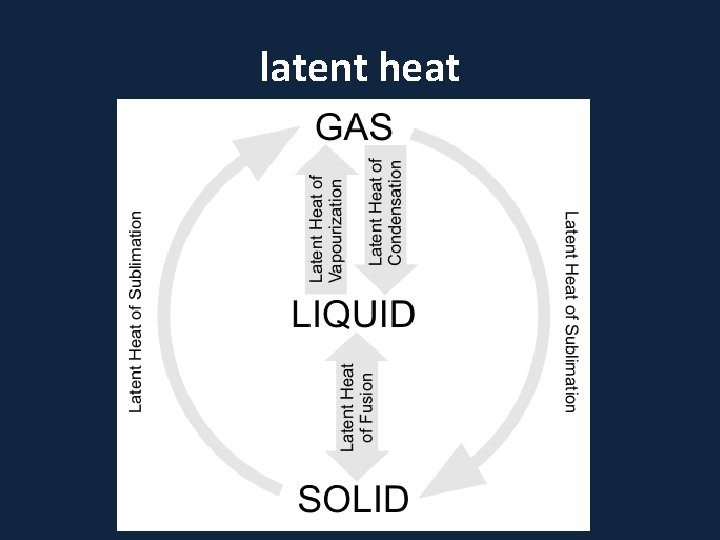
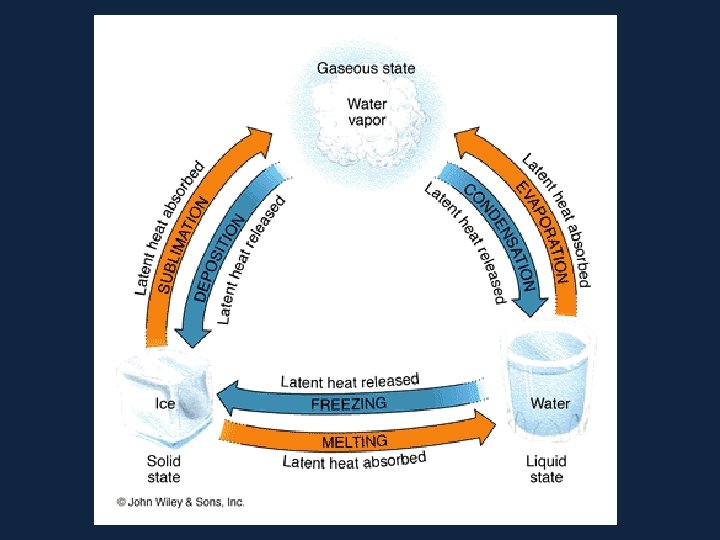
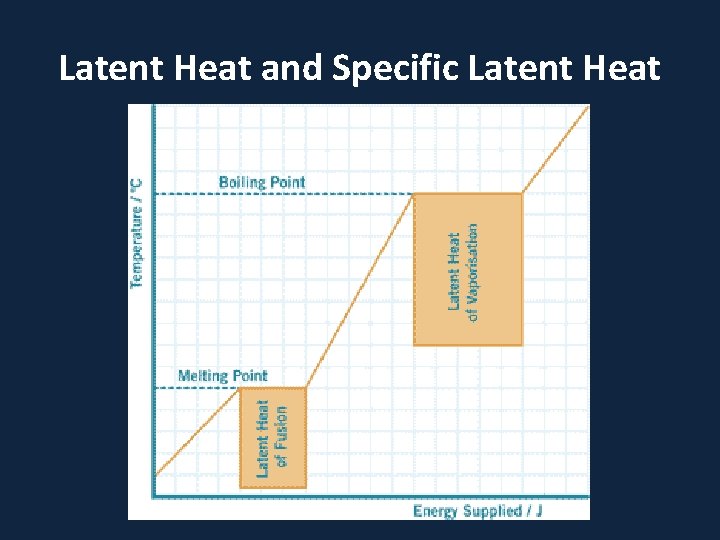
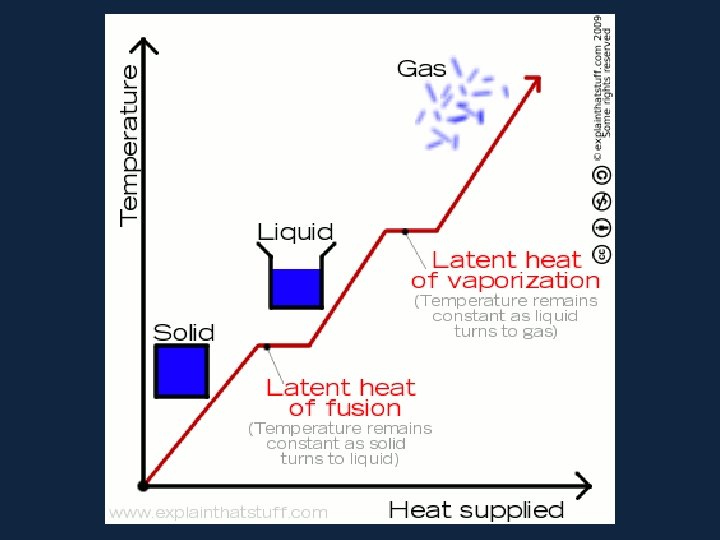
• When a substance changes from one state to another, latent heat is added or releasedปลอยออกมา in the process. • Latent heat: the energy required to change from one state to another at constant temperature

• Consider the water substance: all three phases of water (vapor, ice, and liquid) can be present at the same time • in a thunderstorm, for example, water is changing phases on a continual basis, therefore, latent heat is added or released on a continual basis

• Latent Heats • Consider the water substance: • liquid --> vapor, latent heat of evaporation is added (about 600 cal per gram) • Q: What are some real-world examples of this process? • vapor --> liquid, latent heat of condensation is released • liquid --> ice, latent heat of freezing is released (about 80 cal per gram) • ice --> liquid, latent heat of fusion����� (melting) is added



– Latent Heats flux: heat released or absorbed when water changes state • melting/freezing: 80 cal/gm • evaporation/condensation: 540 cal/gm • condensation at the earth's surface produces dew, fog or frost ������ • sublimation: 677 cal/gm, i. e. 80 cal/gm to solid - liquid and 597 cal/gm for liquid - gas at 0º C – soil heat flux: internal heat flux by conduction in soil and convection in seeping soil water – horizontal heat flux (for water)



latent heat



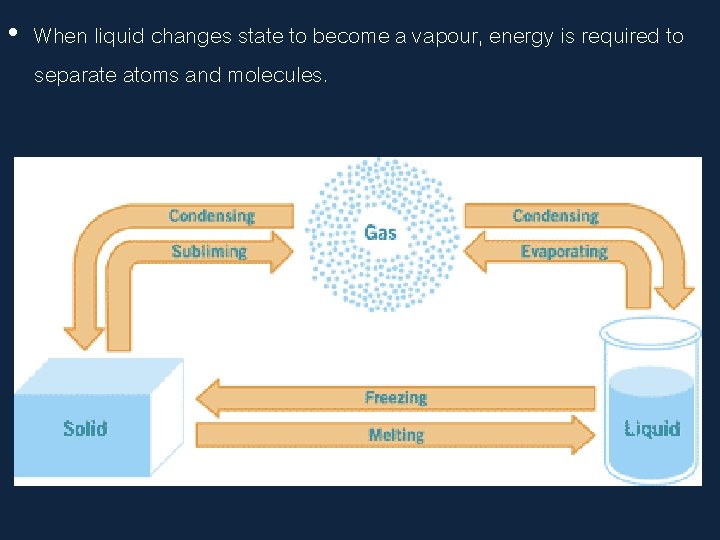
• When liquid changes state to become a vapour, energy is required to separate atoms and molecules.

Latent Heat and Specific Latent Heat



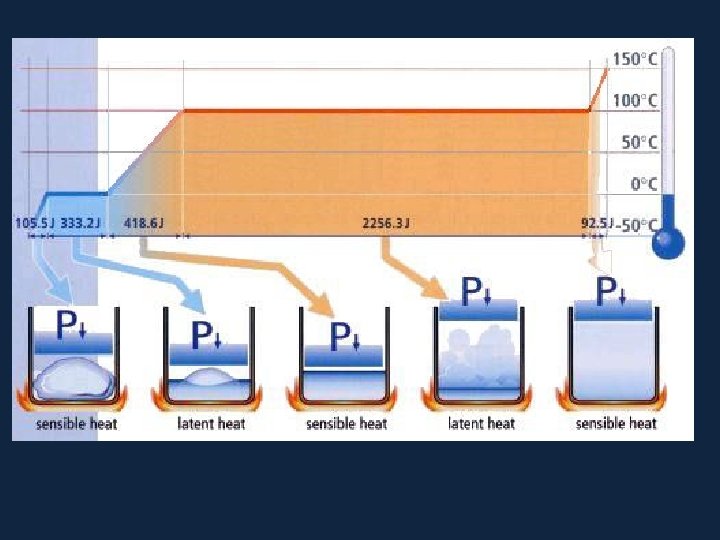
• Sensible heat �������� When an object is heated, its temperature rises as heat is added. The increase in heat is called sensible heat. Similarly, when heat is removed from an object and its temperature falls, the heat removed is also called sensible heat. Heat that causes a change in temperature in an object is called sensible heat.

• All pure substances in nature able to change their state. Solids can become liquids (ice to water) and liquids can become gases (water to vapor) but changes such as these require the addition or removal of heat. The heat that causes these changes is called latent heat.

• Latent heat however, does not affect the temperature of a substance - for example, water remains at 100°C while boiling. The heat added to keep the water boiling is latent heat. Heat that causes a change of state with no change in temperature is called latent heat.
 Changing state
Changing state Vocab
Vocab States of matter
States of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Four states of matter
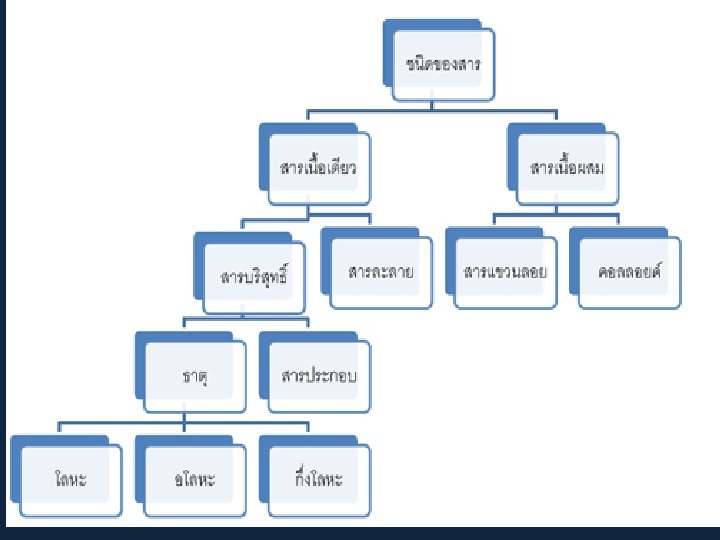
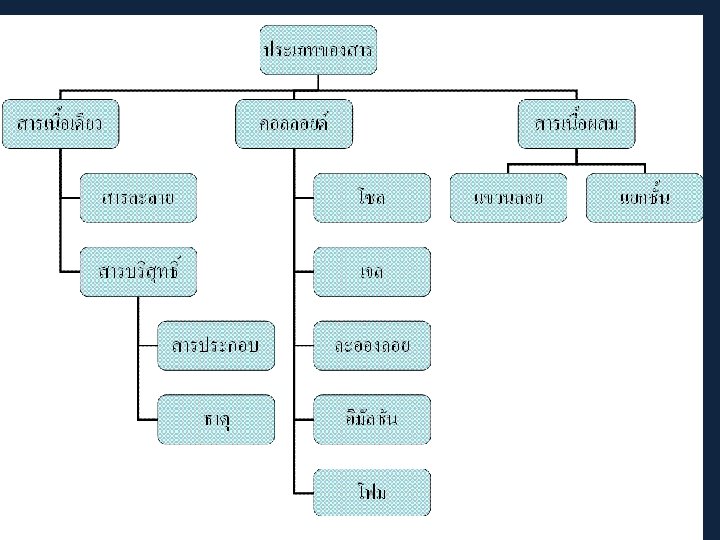
Four states of matter Concept map of states of matter
Concept map of states of matter Phet states of matter basics
Phet states of matter basics Uses of heat
Uses of heat Big states vs small states guard against tyranny
Big states vs small states guard against tyranny Five states of matter
Five states of matter What were the 11 free states
What were the 11 free states Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Four states of matter
Four states of matter Grey vs white matter
Grey vs white matter States of matter: basics
States of matter: basics 5 states of matter
5 states of matter 4 fe
4 fe Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter solid liquid gas
States of matter solid liquid gas Whats the four states of matter
Whats the four states of matter 5 states of matter
5 states of matter The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids States of matter objectives
States of matter objectives Southern states of america
Southern states of america Chemical equation with states of matter
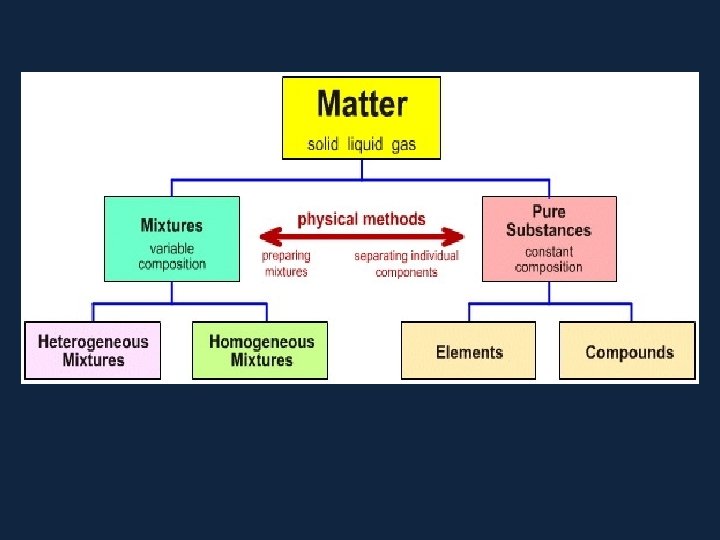
Chemical equation with states of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter States of matter
States of matter States of matter foldable
States of matter foldable Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Four phase changes
Four phase changes Interconversion of states of matter
Interconversion of states of matter What makes up the diencephalon
What makes up the diencephalon States of matter
States of matter Solid liquid and gas diagram
Solid liquid and gas diagram Stayes of matter
Stayes of matter Whats the study of matter and energy
Whats the study of matter and energy Grey matter and white matter in brain
Grey matter and white matter in brain Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Intro to matter
Intro to matter States of matter mind map
States of matter mind map Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Heat thermal energy and temperature
Heat thermal energy and temperature Jeopardy states of matter
Jeopardy states of matter Matter flowchart
Matter flowchart The kinetic theory of matter states that
The kinetic theory of matter states that