CLASSIFYING MATTER Classifying Matter Concept Map Matter Pure



- Slides: 12

CLASSIFYING MATTER

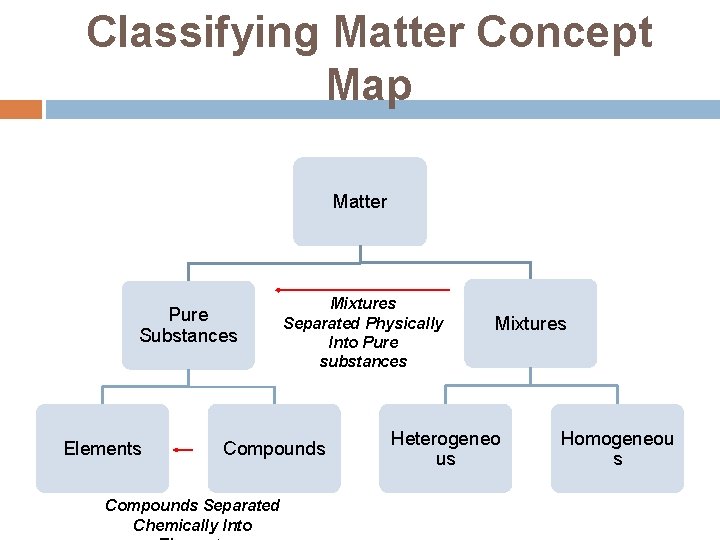
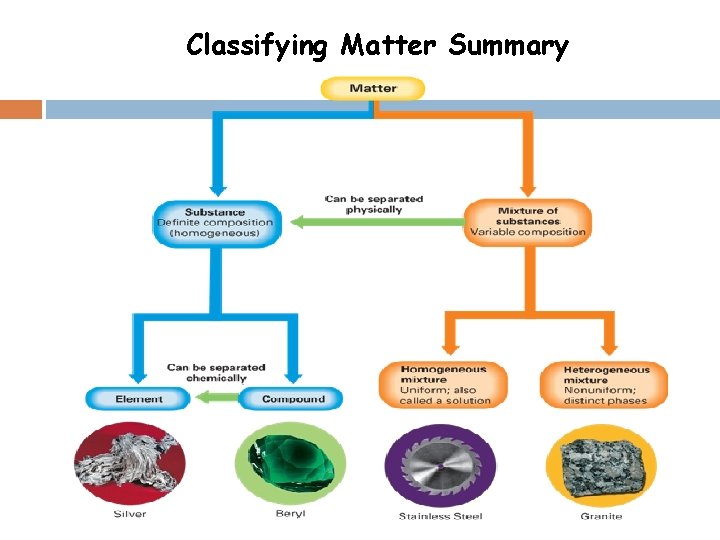
Classifying Matter Concept Map Matter Pure Substances Elements Mixtures Separated Physically Into Pure substances Compounds Separated Chemically Into Mixtures Heterogeneo us Homogeneou s

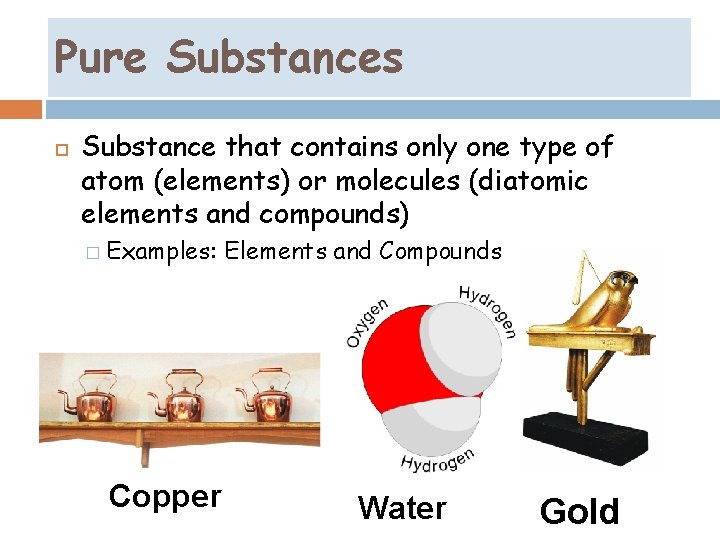
Pure Substances Substance that contains only one type of atom (elements) or molecules (diatomic elements and compounds) � Examples: Copper Elements and Compounds Water Gold

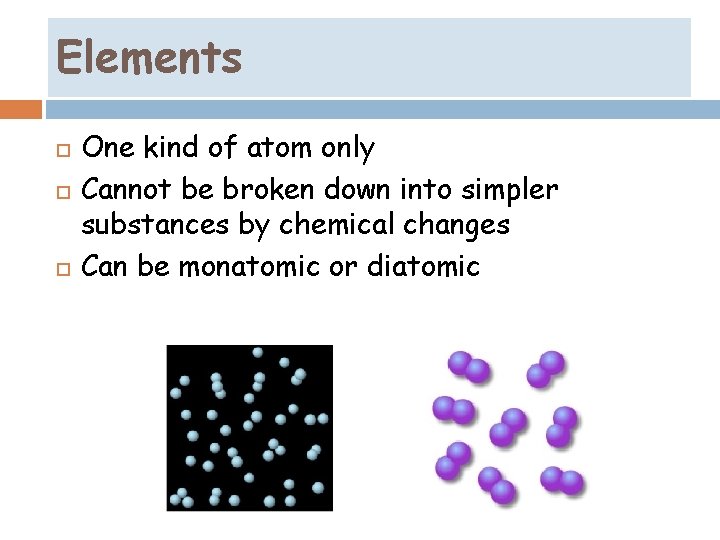
Elements One kind of atom only Cannot be broken down into simpler substances by chemical changes Can be monatomic or diatomic

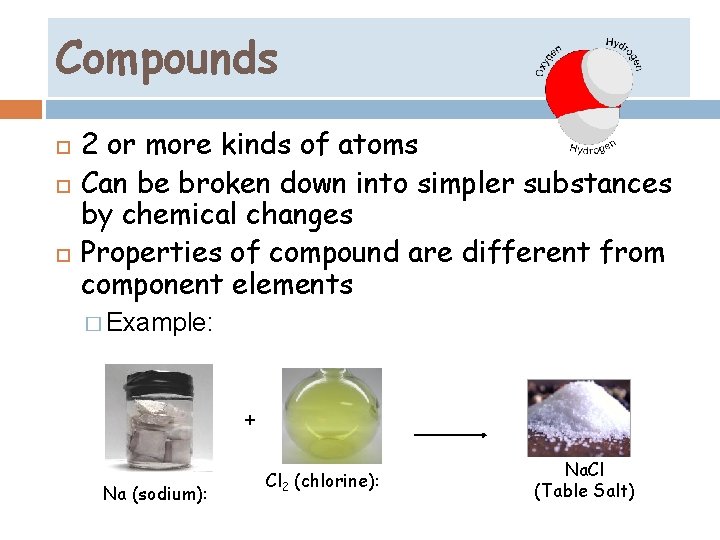
Compounds… 2 or more kinds of atoms Can be broken down into simpler substances by chemical changes Properties of compound are different from component elements � Example: + Na (sodium): Cl 2 (chlorine): Na. Cl (Table Salt)

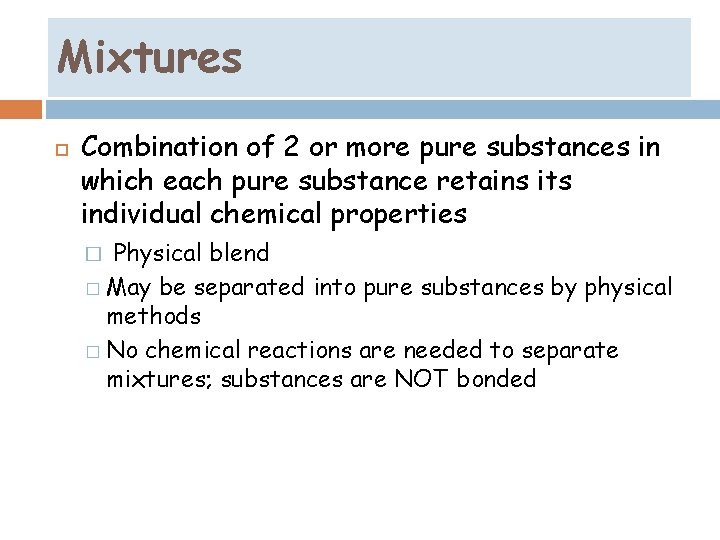
Mixtures Combination of 2 or more pure substances in which each pure substance retains its individual chemical properties Physical blend � May be separated into pure substances by physical methods � No chemical reactions are needed to separate mixtures; substances are NOT bonded �

1. Homogenous Mixtures Mixture in which the composition is uniform; constant composition throughout – always has a single phase �Also called solutions �Ex. Air, Kool-Aid

2. Heterogeneous Mixture A mixture in which the composition is not uniform throughout; does not blend smoothly and in which the individual substances remain distinct

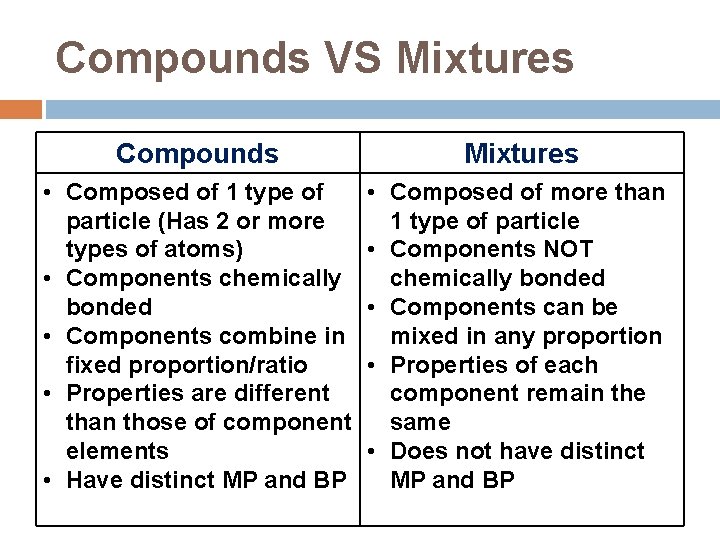
Compounds VS Mixtures Compounds Mixtures • Composed of 1 type of particle (Has 2 or more types of atoms) • Components chemically bonded • Components combine in fixed proportion/ratio • Properties are different than those of component elements • Have distinct MP and BP • Composed of more than 1 type of particle • Components NOT chemically bonded • Components can be mixed in any proportion • Properties of each component remain the same • Does not have distinct MP and BP

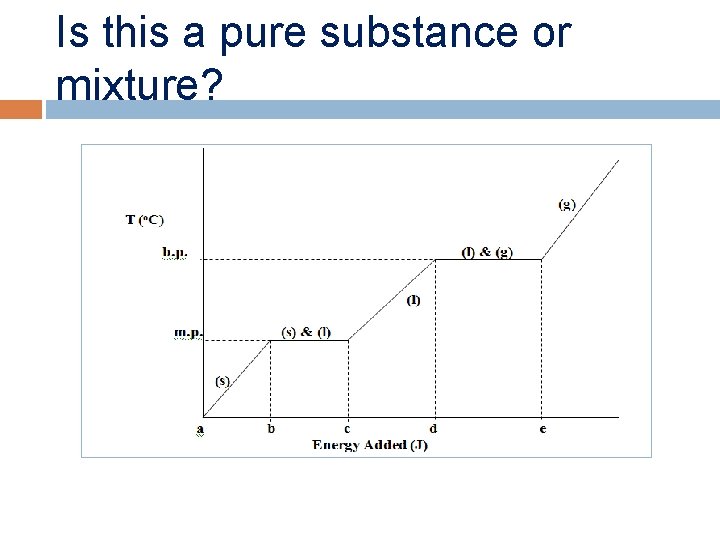
Is this a pure substance or mixture?

Classifying Matter Summary

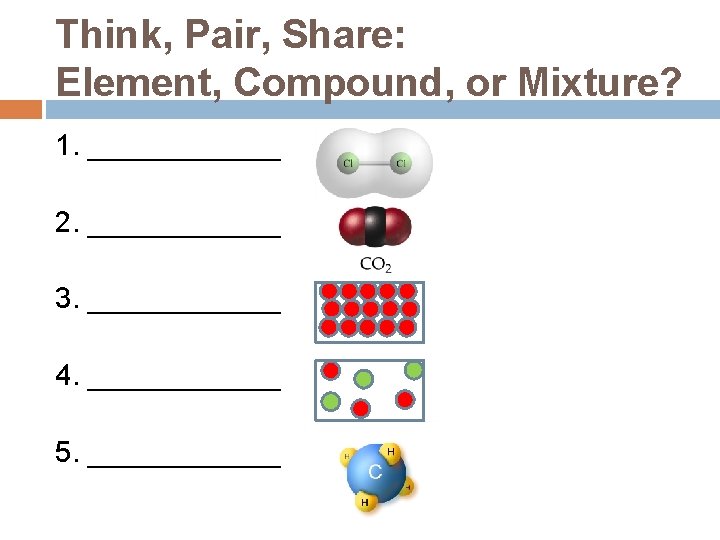
Think, Pair, Share: Element, Compound, or Mixture? 1. ______ 2. ______ 3. ______ 4. ______ 5. ______