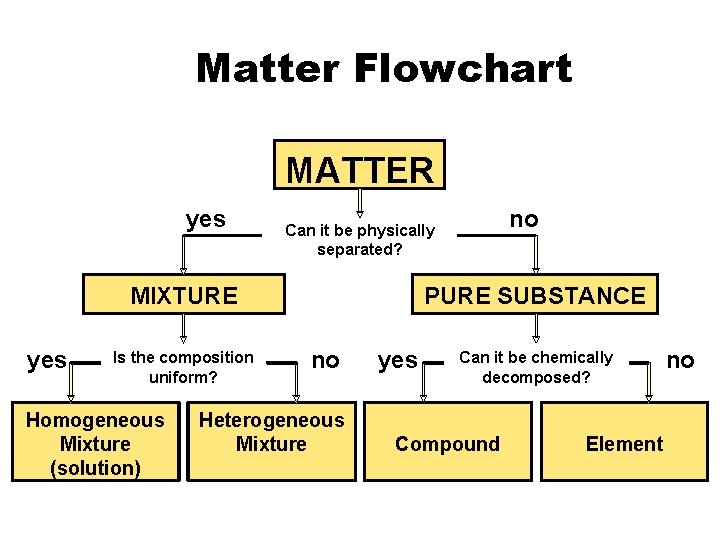
Matter Flowchart MATTER yes MIXTURE yes Is the









- Slides: 15


Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound Element no

Mixtures • Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

Pure Substances • Element – composed of identical atoms – EX: copper wire, aluminum foil

Pure Substances • Compound – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl)

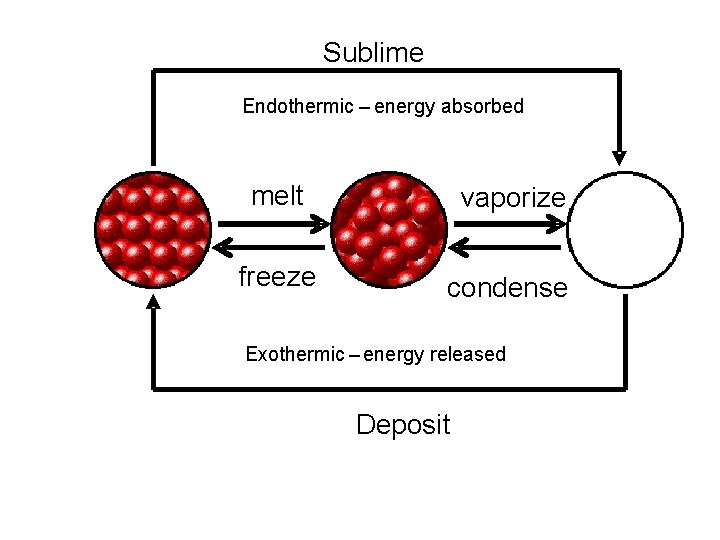
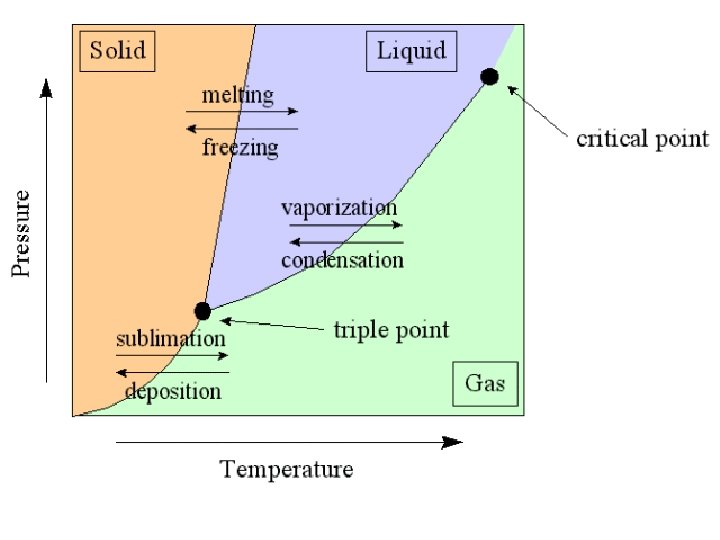
Sublime Endothermic – energy absorbed melt vaporize freeze condense Exothermic – energy released Deposit

Physical Properties of Matter Color, shape, size, etc. Also, the following properties located on your reference tables: • • Melting Points Boiling Points Density Solubility Chemical Properties refer to the ability of a substance to combine or react with other substances. Ex. : Copper turns green (think Statue of Liberty)

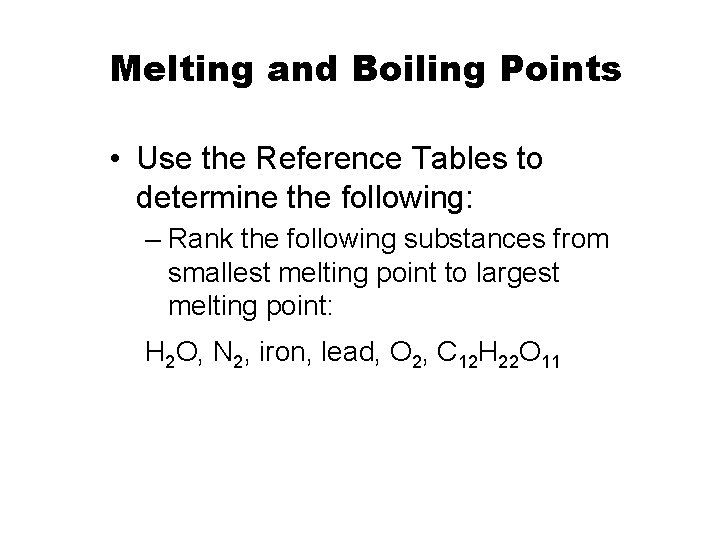
Melting and Boiling Points • Use the Reference Tables to determine the following: – Rank the following substances from smallest melting point to largest melting point: H 2 O, N 2, iron, lead, O 2, C 12 H 22 O 11

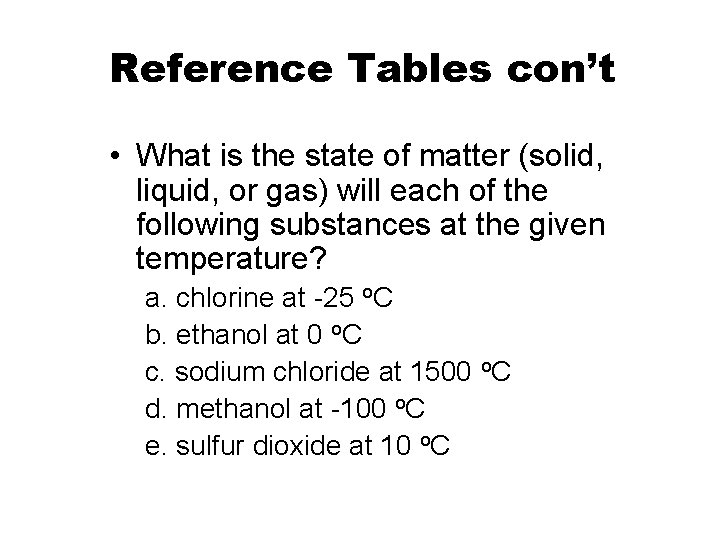
Reference Tables con’t • What is the state of matter (solid, liquid, or gas) will each of the following substances at the given temperature? a. chlorine at -25 o. C b. ethanol at 0 o. C c. sodium chloride at 1500 o. C d. methanol at -100 o. C e. sulfur dioxide at 10 o. C

Density • Using the reference tables, determine the following: – Rank the following substances from greatest density to smallest density: H 2 O, Mg, C 6 H 14, Pb, methanol, Na. Cl

Reference Tables con’t • What is the mass of 36. 5 m. L of ethanol? • What is the volume of 50. 0 g of molten lead? • You have 100. 0 cm 3 of glucose and sucrose. Which sample has the greatest mass? Support answer with calculations.

Physical Changes • Changes of state (melting, boiling, freezing, etc. ) • Color change Other physical changes include: -change in shape or size Chemical Change: -results in the formation of a new or different substance -ex. : rusting, burning, color changes, gas formation


