Chemistry Exploring Matter Classifying Matter Classifying Matter Chart








































- Slides: 46

Chemistry: Exploring Matter Classifying Matter

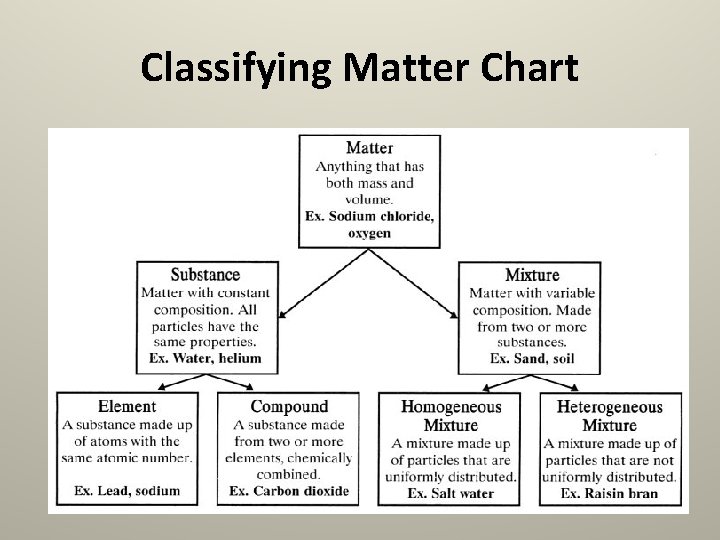
Classifying Matter Chart

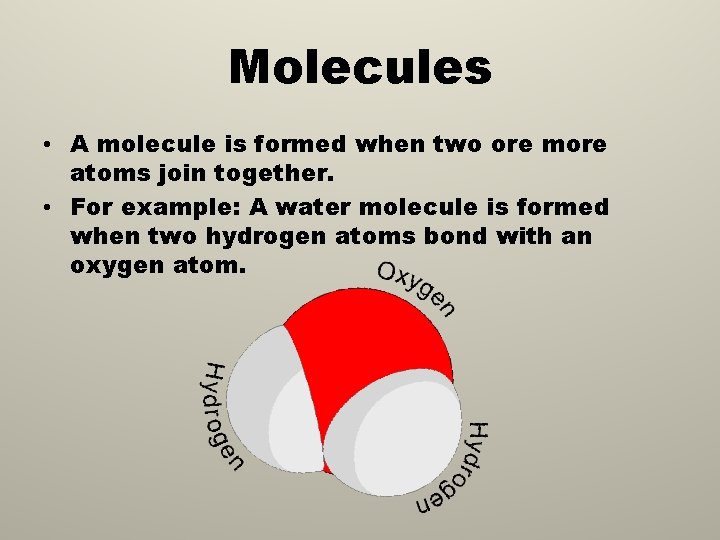
Molecules • A molecule is formed when two ore more atoms join together. • For example: A water molecule is formed when two hydrogen atoms bond with an oxygen atom.

Pure Substance • A Pure Substance is matter that has definite chemical and physical properties. • It is made of only one kind of particle. Aluminum Foil is made of only aluminum molecules. Sugar is made of only sugar molecules.

Pure Substance • Pure Substances come in two types: Elements GOLD & Compounds WATER

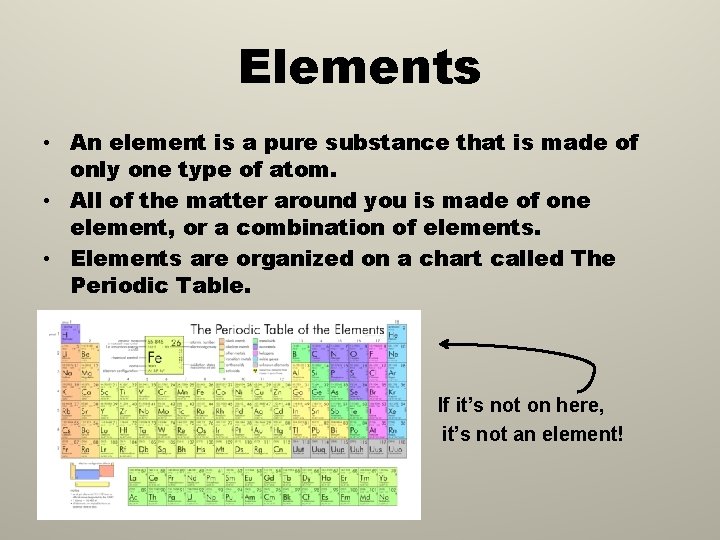
Elements • An element is a pure substance that is made of only one type of atom. • All of the matter around you is made of one element, or a combination of elements. • Elements are organized on a chart called The Periodic Table. • • If it’s not on here, it’s not an element!

Compounds • A compound is a pure substance made of two or more elements that are chemically combined. • Water is an example of a compound. It is made of two types of atoms: Hydrogen and Oxygen. – But these atoms are chemically bonded together in one H 20 particle.

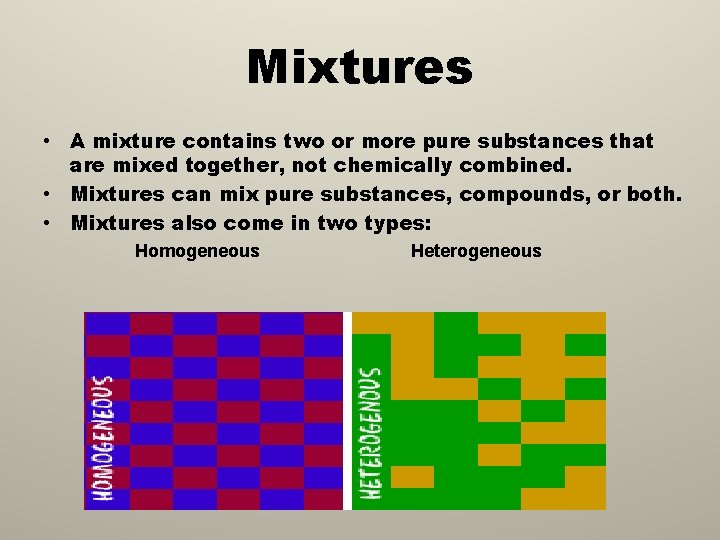
Mixtures • A mixture contains two or more pure substances that are mixed together, not chemically combined. • Mixtures can mix pure substances, compounds, or both. • Mixtures also come in two types: Homogeneous Heterogeneous

Homogeneous • In a homogeneous mixture, the different substances are mixed very thoroughly. • Sometimes they are mixed so well you cannot tell that there is more than one substance in the mixture.

Heterogeneous • In a heterogeneous mixture, you can see the different substances that have been combined. • A heterogeneous substance is not mixed evenly, it is not the same throughout. You can see all of the different parts.

Solutions • A solution is the name for homogenous mixture that is so well mixed, you can no longer see the different substances. • Solutions have two parts: Solvent & Solute

Solvent • The solvent in a solution is what you have the most of. This is what you dissolve another substance in. • For example: – If you are dissolving sugar in to a glass of iced tea – the iced tea is the solvent. You have much more tea than sugar.

Solute • The solute in a solution is the substance you have the least of. This is the substance that gets dissolved. • For example: – In our iced tea example, sugar is the solute. You have far less sugar than iced tea.

Solubility • Solubility is a measure of how well a solute can dissolve in a solvent at a given temperature. • The solubility of a substance tells you how much of it you can add before it will not be able to dissolve any more.

Solubility • Think About It: – You are making homemade chocolate milk with milk and chocolate syrup. – If you add too much syrup to the milk, when you finish there will be chocolate syrup stuck to the bottom of the glass. – This is because the milk can only have so much syrup dissolved into it before it can’t take any more.

Can you identify the following? Tell me if the item in the photo is an element, compound, or mixture. • element : contains just one type of atom. • compound : contains two or more different atoms chemically joined together. • mixture : contains two or more different substances that are only physically joined together, not chemically.

Element, Compound, or Mixture? Rocks

Element, Compound, or Mixture? Rocks

Element, Compound, or Mixture? Copper

Element, Compound, or Mixture? Copper

Element, Compound, or Mixture? Jelly Beans

Element, Compound, or Mixture? Jelly Beans

Element, Compound, or Mixture? Table Sugar

Element, Compound, or Mixture? Table Sugar

Element, Compound, or Mixture? Diamond

Element, Compound, or Mixture? Diamond

Element, Compound, or Mixture? Tea

Element, Compound, or Mixture? Tea

Element, Compound, or Mixture? Salt

Element, Compound, or Mixture? Salt

Element, Compound, or Mixture? Neon Gas

Element, Compound, or Mixture? Neon Gas

Element, Compound, or Mixture? Salad

Element, Compound, or Mixture? Salad

Element, Compound, or Mixture? Pure Water

Element, Compound, or Mixture? Pure Water

Element, Compound, or Mixture? Aluminum

Element, Compound, or Mixture? Aluminum

Element, Compound, or Mixture? Lemonade

Element, Compound, or Mixture? Lemonade

Element, Compound, or Mixture? Silver

Element, Compound, or Mixture? Silver

Element, Compound, or Mixture? Sand

Element, Compound, or Mixture? Sand

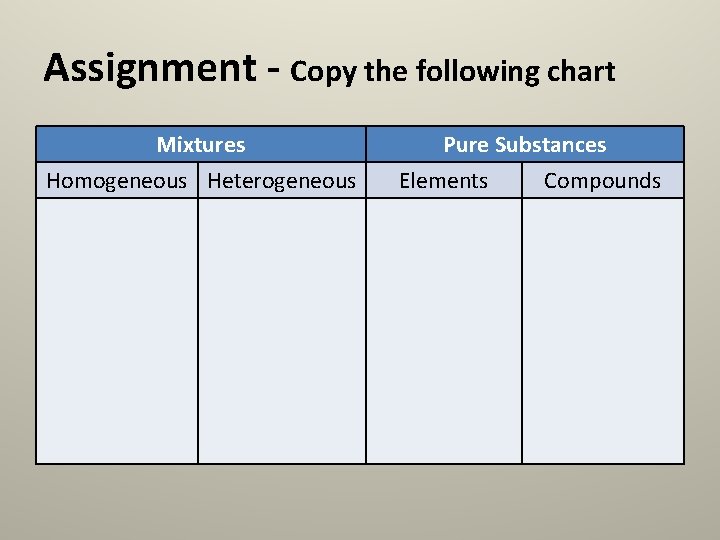
Assignment - Copy the following chart Mixtures Homogeneous Heterogeneous Pure Substances Elements Compounds

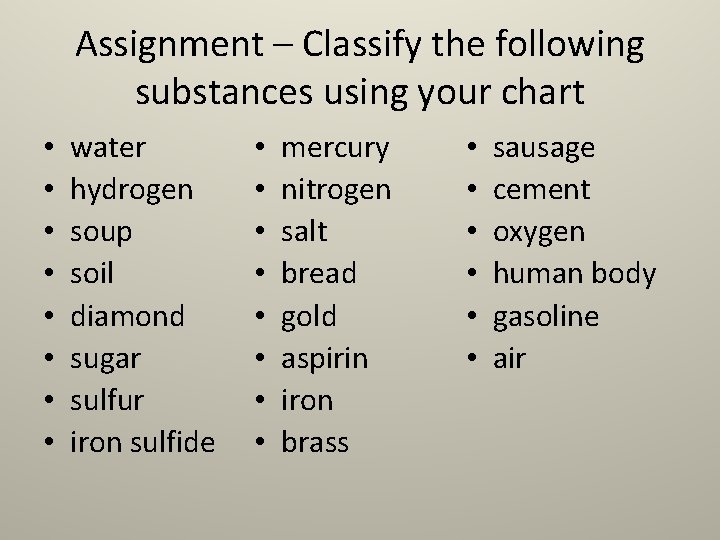
Assignment – Classify the following substances using your chart • • water hydrogen soup soil diamond sugar sulfur iron sulfide • • mercury nitrogen salt bread gold aspirin iron brass • • • sausage cement oxygen human body gasoline air