Chapter 2 MATTER 1 Section 1 CLASSIFYING MATTER





































- Slides: 70

Chapter 2 MATTER 1

Section 1 CLASSIFYING MATTER 2

What is matter? Matter: anything that has mass and takes up space Any materials you can hold or touch! Even air!!! 3

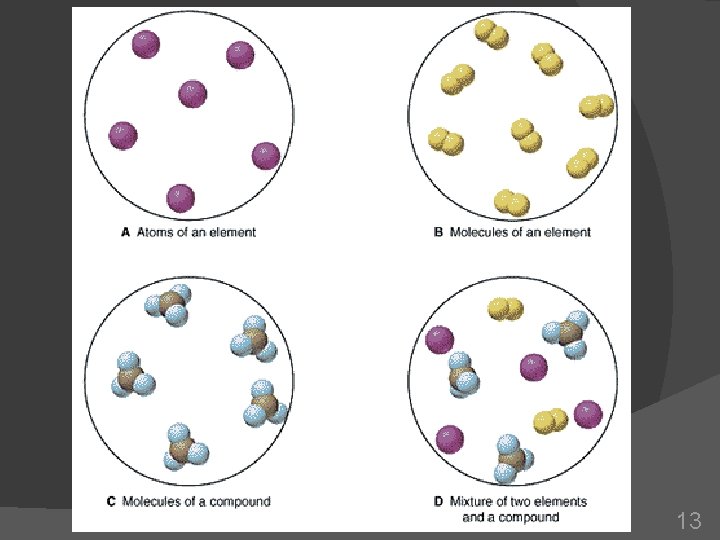
How can matter be classified? Classifying matter is based on what makes up the matter. 3 groups: Elements Compounds Mixtures 4

Can elements be broken down into simpler substances? Element: substance that can’t be broken down or separated by chemical means Made up of one kind of atom Atom: smallest unit of an element that keeps the element’s chemical properties 5

Can elements be broken down into simpler substances? Represented by symbols that are usually one or two letters O = Oxygen H = Hydrogen Ca = Calcium Pb = Lead 6

Periodic Table lists all the elements and symbols Molecule: combination of two or elements that are combined in a definite ratio 7

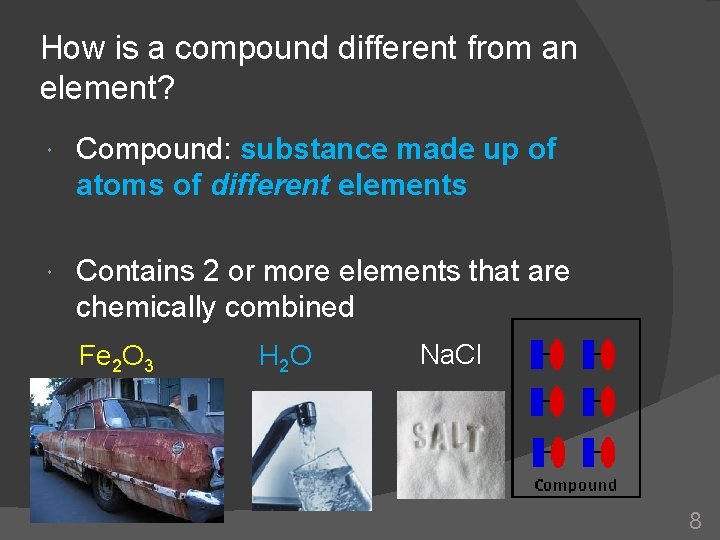
How is a compound different from an element? Compound: substance made up of atoms of different elements Contains 2 or more elements that are chemically combined Fe 2 O 3 H 2 O Na. Cl 8

When elements combine in a compound, they always combine in the same proportions! H 2 O is always H 2 O 2 is NOT H 2 O 9

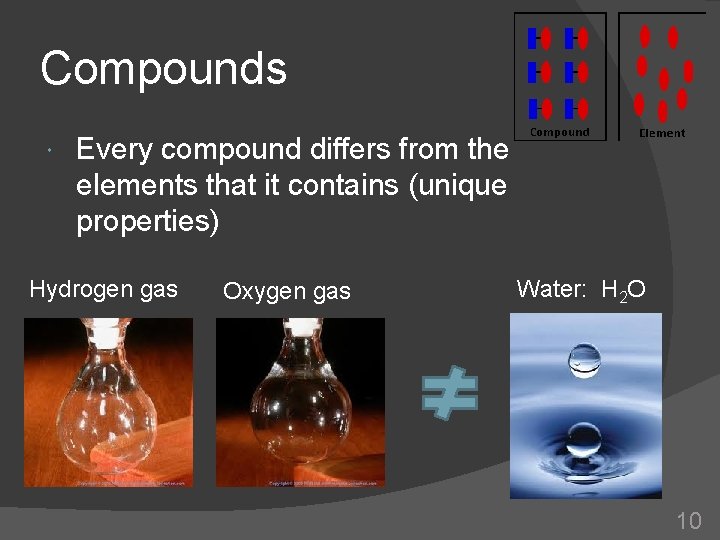
Compounds Every compound differs from the elements that it contains (unique properties) Hydrogen gas Oxygen gas Water: H 2 O 10

Chemical formulas are used to represent compounds Shows how many atoms of each element are in a substance The number of atoms of each element is written as a subscript after the elements symbol 11

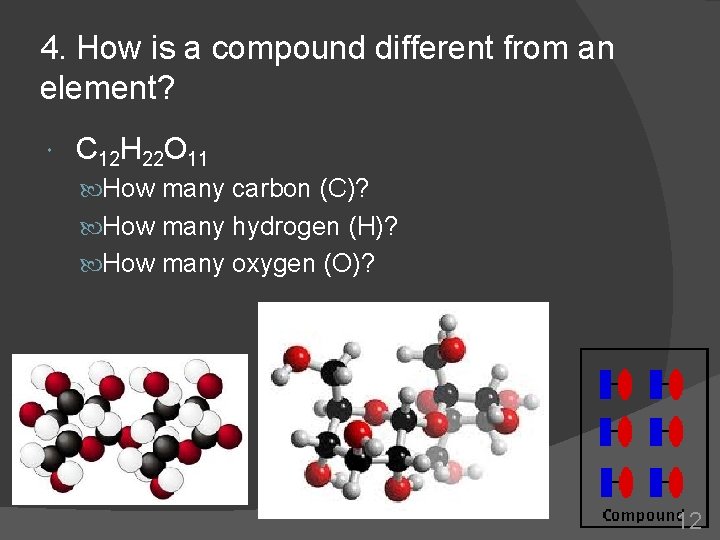
4. How is a compound different from an element? C 12 H 22 O 11 How many carbon (C)? How many hydrogen (H)? How many oxygen (O)? 12

13

Pure Substances and Mixtures Pure Substance: matter that has a fixed composition and definite properties 14

Mixture: combination of two or more pure substances that are not chemically combined 15

Elements and Compounds are pure substances because they are chemically combined and can’t be physically separated Mixtures can be physically separated into parts. Parts of pure substances are chemically combined and can’t be physically separated. 16

Classify mixtures on how well substances mix. Homogeneous Mixture: components are evenly distributed 17

Classify mixtures on how well substances mix. Heterogeneous Mixture: substances that are not evenly distributed 18

Miscible and Immiscible Miscible: homogeneous mixture made up of many different liquids that can be mixed and stay mixed Example: Vinegar 19

Miscible and Immiscible: Mixture that does not mix or will not stay mixed. Example: Oil and Water 20

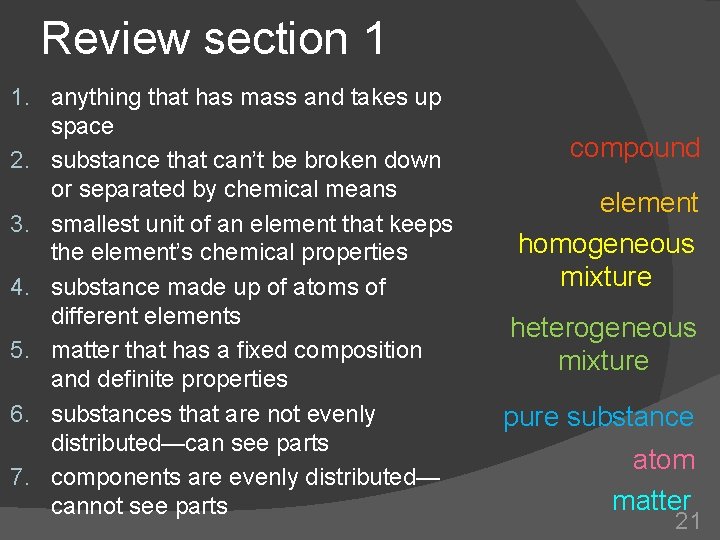
Review section 1 1. anything that has mass and takes up space 2. substance that can’t be broken down or separated by chemical means 3. smallest unit of an element that keeps the element’s chemical properties 4. substance made up of atoms of different elements 5. matter that has a fixed composition and definite properties 6. substances that are not evenly distributed—can see parts 7. components are evenly distributed— cannot see parts compound element homogeneous mixture heterogeneous mixture pure substance atom matter 21

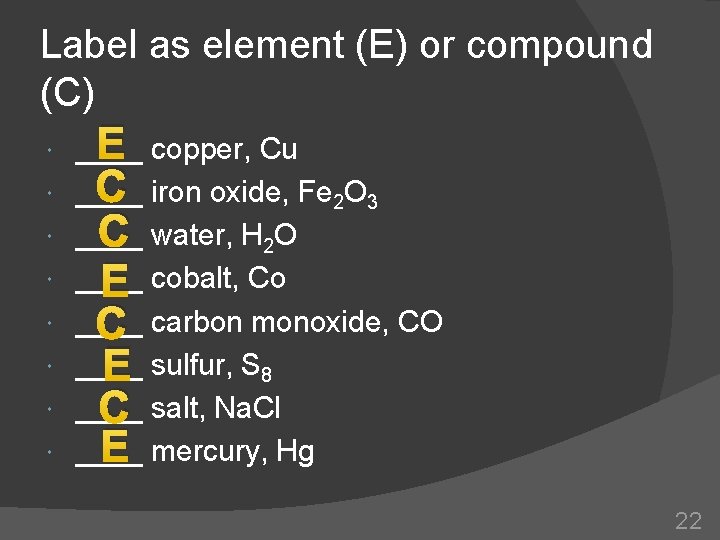
Label as element (E) or compound (C) E copper, Cu ____ C iron oxide, Fe 2 O 3 ____ C water, H 2 O ____ E cobalt, Co ____ C carbon monoxide, CO ____ E sulfur, S 8 ____ C salt, Na. Cl E mercury, Hg ____ 22

Label as heterogeneous mixture (E) or homogeneous mixture (O) ____ a salad ____ salt water ____ Kool aide ____ vinegar ____ chocolate syrup ____ banana split ____ human blood ____ muddy water 23

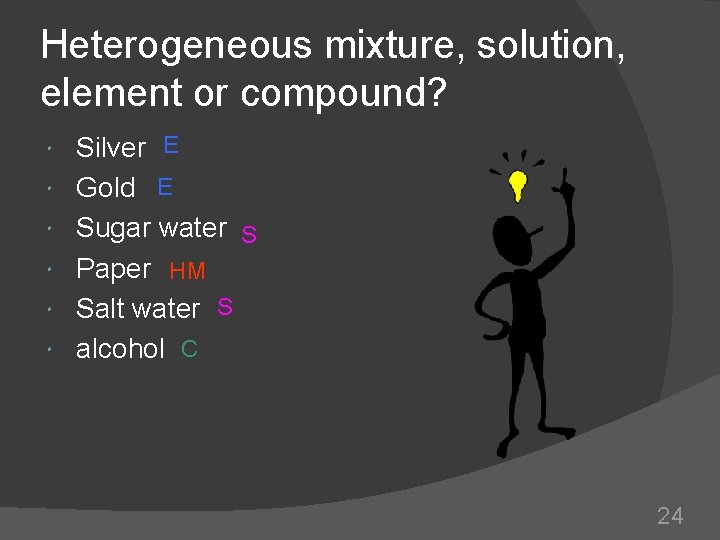
Heterogeneous mixture, solution, element or compound? Silver E Gold E Sugar water S Paper HM Salt water S alcohol C 24

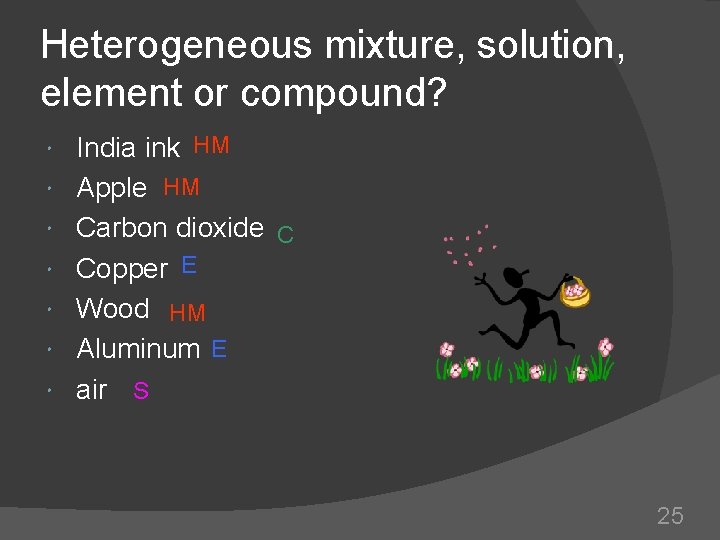
Heterogeneous mixture, solution, element or compound? India ink HM Apple HM Carbon dioxide C Copper E Wood HM Aluminum E air S 25

Heterogeneous mixture, solution, element or compound? Salt C Italian salad dressing HM Hydrogen E Hot tea with sugar in it S Cold tea with sugar in HM it Milk HM oxygen E 26

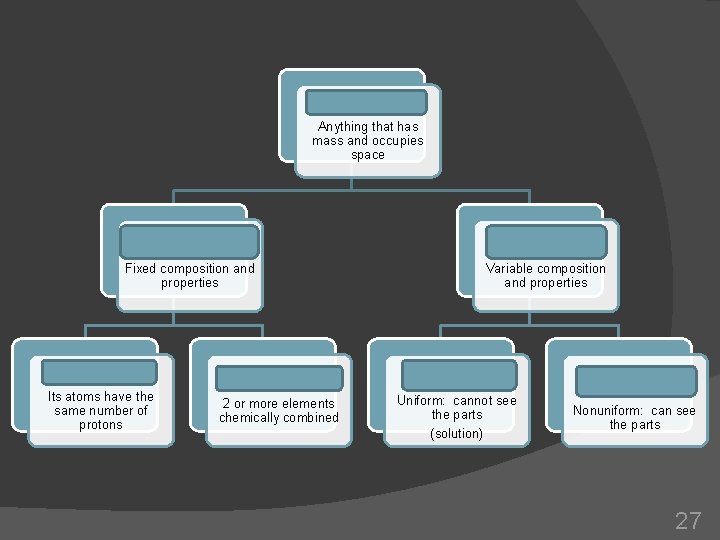
Matter Anything that has mass and occupies space Pure substances Fixed composition and properties Element Its atoms have the same number of protons Compound 2 or more elements chemically combined Mixture Variable composition and properties Homogeneous Mixture Uniform: cannot see the parts (solution) Heterogeneous Mixture Nonuniform: can see the parts 27

Section 2 PROPERTIES OF MATTER 28

7. Why are color, volume, and density classified as physical properties? Because physical properties are defined as characteristics that can be observed without changing the identity of the substance For example: Shape Color Odor Texture 29

8. What are 3 ways physical properties can help us classify substances? Can help identify substances It is cold and wet—ice cream It is red, so STOP at the stop light!!! Can be observed and measured using our senses State of matter Melting point, boiling point Strength Hardness Magnetism 30

8. What are 3 ways physical properties can help us classify substances? Help determine uses Ability to conduct heat or electricity—then we can make pots and pans to use for cooking 31

Summary: Physical properties Shape Color Odor Texture State of matter Melting point Boiling point Strength Hardness Magnetism Ability to conduct heat Ability to conduct electricity 32

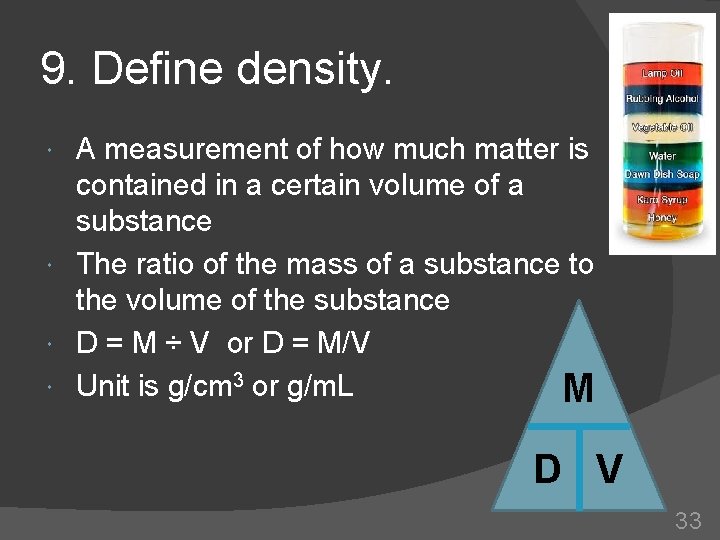
9. Define density. A measurement of how much matter is contained in a certain volume of a substance The ratio of the mass of a substance to the volume of the substance D = M ÷ V or D = M/V Unit is g/cm 3 or g/m. L M D V 33

10. How is density different from weight? Density is mass/volume Weight is a measure of the pull of gravity on an object Weight can change e. g. on the moon an object will weigh less than on earth Density does not change on the moon— the same as here on earth 34

Density problems M D V 35

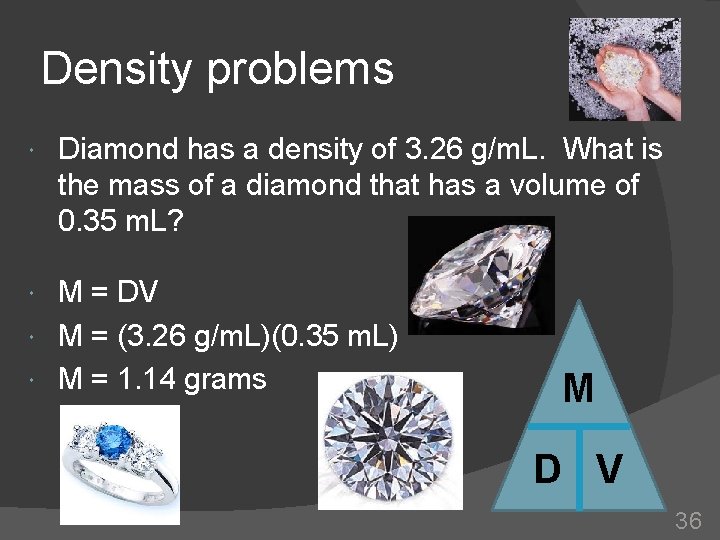
Density problems Diamond has a density of 3. 26 g/m. L. What is the mass of a diamond that has a volume of 0. 35 m. L? M = DV M = (3. 26 g/m. L)(0. 35 m. L) M = 1. 14 grams M D V 36

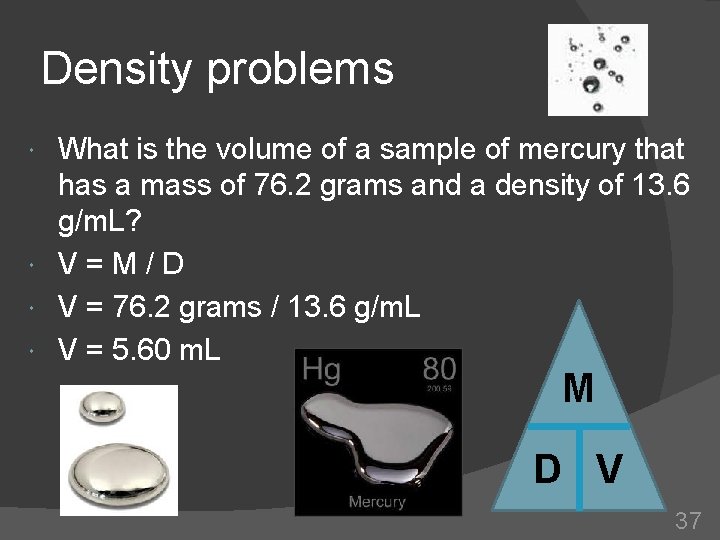
Density problems What is the volume of a sample of mercury that has a mass of 76. 2 grams and a density of 13. 6 g/m. L? V=M/D V = 76. 2 grams / 13. 6 g/m. L V = 5. 60 m. L M D V 37

Density problems Do the sheet of density problems. Show work, just like my previous slides!!! Make sure each answer has a correct unit: Density is g/m. L or g/cm 3 Mass is grams Volume is m. L or cm 3 M D V 38

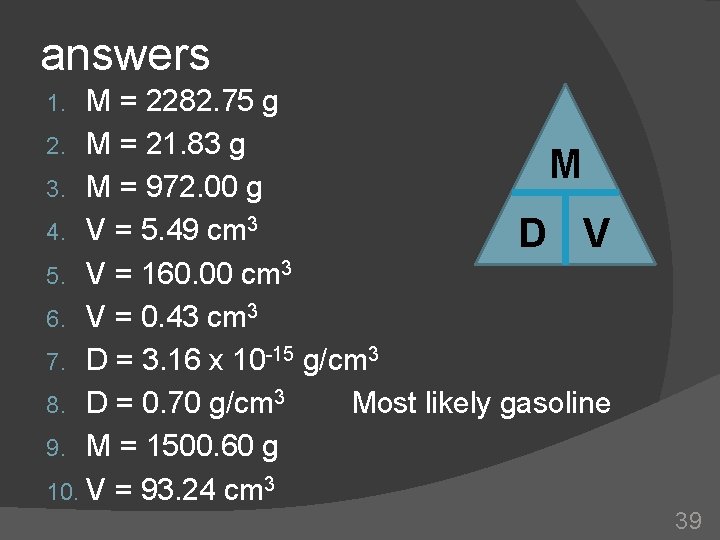
answers M = 2282. 75 g 2. M = 21. 83 g M 3. M = 972. 00 g 4. V = 5. 49 cm 3 D V 5. V = 160. 00 cm 3 6. V = 0. 43 cm 3 7. D = 3. 16 x 10 -15 g/cm 3 8. D = 0. 70 g/cm 3 Most likely gasoline 9. M = 1500. 60 g 10. V = 93. 24 cm 3 1. 39

Density problems 1) M = DV The largest ruby in the world is 10. 9 cm long, M = (3. 97)(575) 9. 10 cm wide, and 5. 80 M = 2282. 75 g cm thick, giving it an overall volume of 575 cm 3. If the density of ruby – a form of aluminum oxide – is 3. 97 g/cm 3, what is the mass of the largest ruby? M D V 40

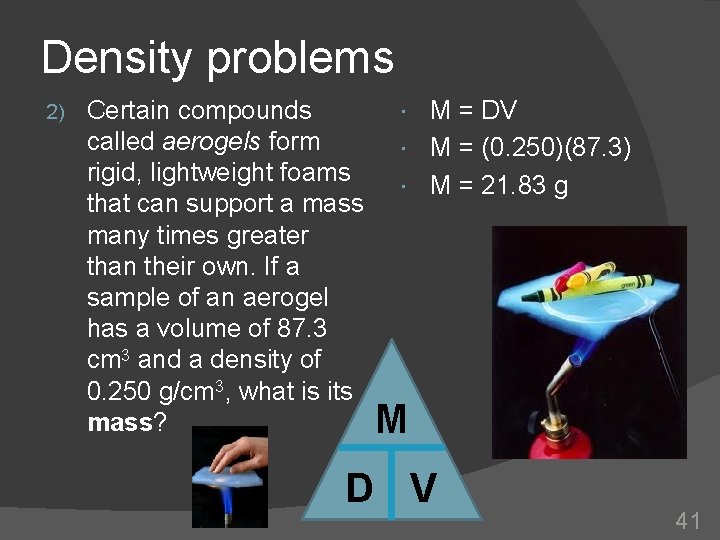
Density problems 2) Certain compounds called aerogels form rigid, lightweight foams that can support a mass many times greater than their own. If a sample of an aerogel has a volume of 87. 3 cm 3 and a density of 0. 250 g/cm 3, what is its mass? M = DV M = (0. 250)(87. 3) M = 21. 83 g M D V 41

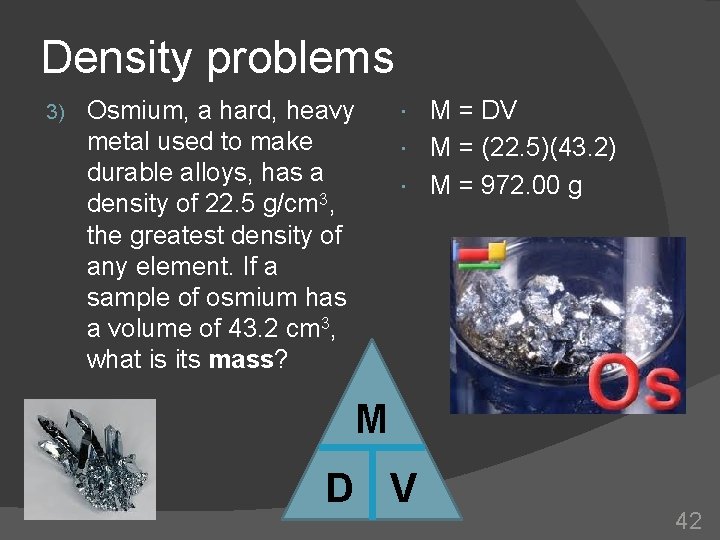
Density problems 3) M = DV M = (22. 5)(43. 2) M = 972. 00 g Osmium, a hard, heavy metal used to make durable alloys, has a density of 22. 5 g/cm 3, the greatest density of any element. If a sample of osmium has a volume of 43. 2 cm 3, what is its mass? M D V 42

Density problems 4) Magnesium is a fairly light V = M / D metal that is combined V = (9. 56) / (1. 74) with other elements to V = 5. 49 cm 3 form lightweight alloys for use in airplanes. The big advantage of magnesium is that it has a relatively low density of 1. 74 g/cm 3. If a sample of magnesium has a mass of 9. 56 g, what is its volume? M D V 43

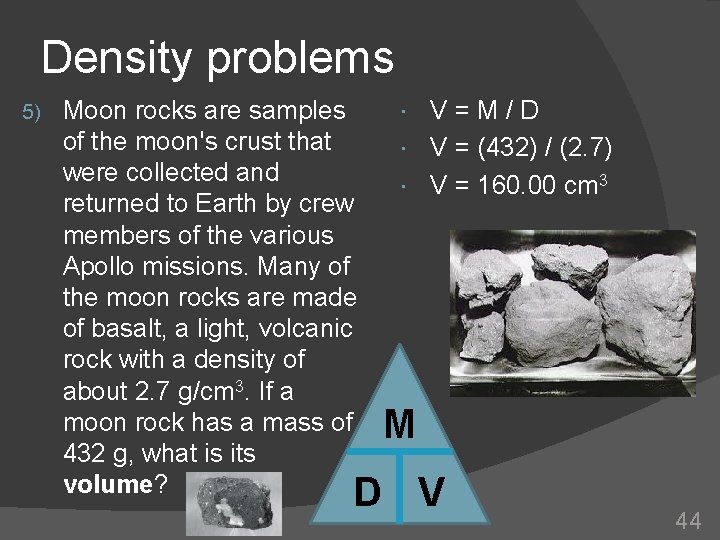
Density problems 5) Moon rocks are samples of the moon's crust that were collected and returned to Earth by crew members of the various Apollo missions. Many of the moon rocks are made of basalt, a light, volcanic rock with a density of about 2. 7 g/cm 3. If a moon rock has a mass of 432 g, what is its volume? V=M/D V = (432) / (2. 7) V = 160. 00 cm 3 M D V 44

Density problems 6) Although both diamond and graphite consist of pure carbon, they have very different densities. This is because of differences in the way the carbon atoms in each substance arranged. If you had a diamond with a mass of 1. 5 g and a density of 3. 51 g/cm 3, what would its volume be? V=M/D V = (1. 5) / (3. 51) V = 0. 43 cm 3 M D V 45

Density problems 7) Outer space is often described as a vacuum, but there is always some matter present. In the space 300 km above Earth's surface, there is as little as 1. 58 x 10 -12 g of matter in a 500. 0 cm 3 volume of space. Based on this data, what is the density of the matter in space? D=M/V D = 1. 58 x 10 -12 5. 0 x 102 Subtract exponents! D = 0. 316 x 10 -14 g/cm 3 D = 3. 16 x 10 -15 g/cm 3 M D V 46

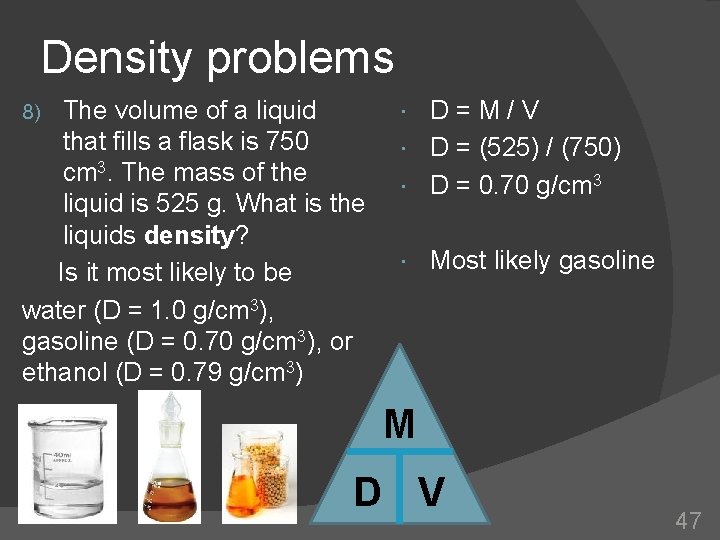
Density problems The volume of a liquid that fills a flask is 750 cm 3. The mass of the liquid is 525 g. What is the liquids density? Is it most likely to be water (D = 1. 0 g/cm 3), gasoline (D = 0. 70 g/cm 3), or ethanol (D = 0. 79 g/cm 3) 8) D=M/V D = (525) / (750) D = 0. 70 g/cm 3 Most likely gasoline M D V 47

Density problems 9) Because inland seas like the Caspian Sea or the Great Salt Lake evaporate faster than they can be refilled, they have higher concentrations of salts than oceans have. The highest concentration of salts in any body of water is found in the Dead Sea, in Israel. If you had 1230 cm 3 of this water, which has a density of 1. 22 g/cm 3, what would be its mass? M = DV M = (1. 22)(1230) M = 1500. 60 g M D V 48

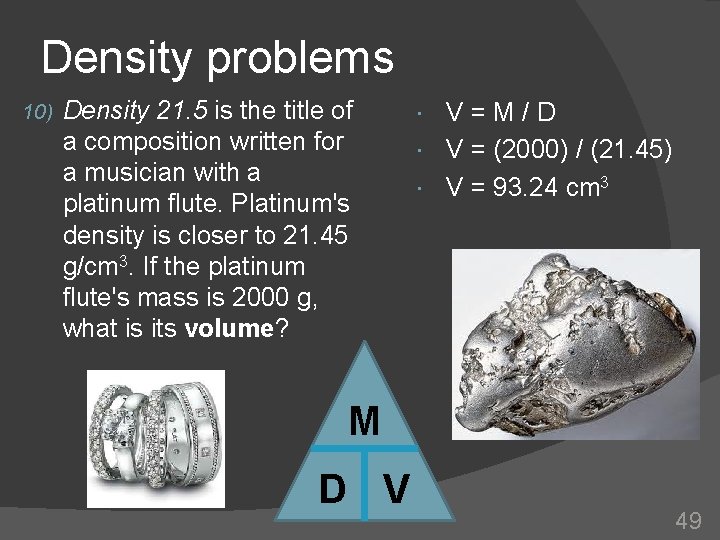
Density problems 10) Density 21. 5 is the title of a composition written for a musician with a platinum flute. Platinum's density is closer to 21. 45 g/cm 3. If the platinum flute's mass is 2000 g, what is its volume? V=M/D V = (2000) / (21. 45) V = 93. 24 cm 3 M D V 49

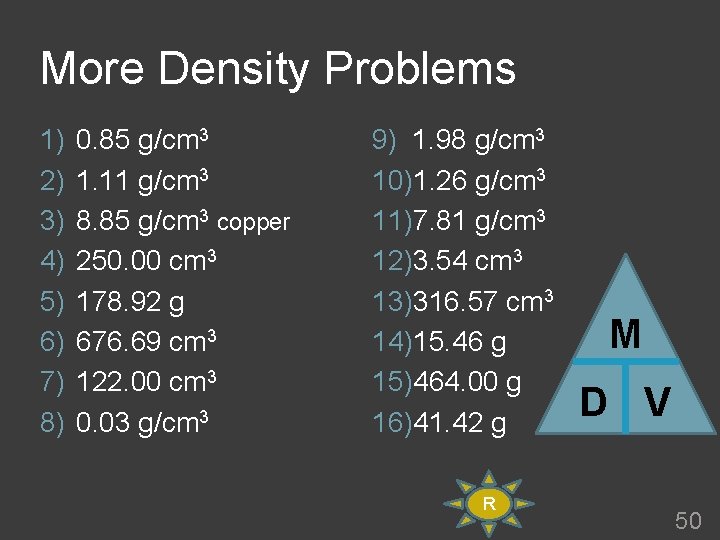
More Density Problems 1) 2) 3) 4) 5) 6) 7) 8) 0. 85 g/cm 3 1. 11 g/cm 3 8. 85 g/cm 3 copper 250. 00 cm 3 178. 92 g 676. 69 cm 3 122. 00 cm 3 0. 03 g/cm 3 9) 1. 98 g/cm 3 10)1. 26 g/cm 3 11)7. 81 g/cm 3 12)3. 54 cm 3 13)316. 57 cm 3 14)15. 46 g 15)464. 00 g 16)41. 42 g R M D V 50

11. Why are flammability and reactivity classified as chemical properties? Chemical properties describe how a substance changes into a new substance, either by combining with other elements or by breaking apart into new substances Reactivity is the capacity of a substance to combine chemically with another substance 51

11. Why are flammability and reactivity classified as chemical properties? Chemical properties describe how a substance changes into a new substance, either by combining with other elements or by breaking apart into new substances Flammability is the ability to burn—or the ability to combine with oxygen in the air s. 3 52

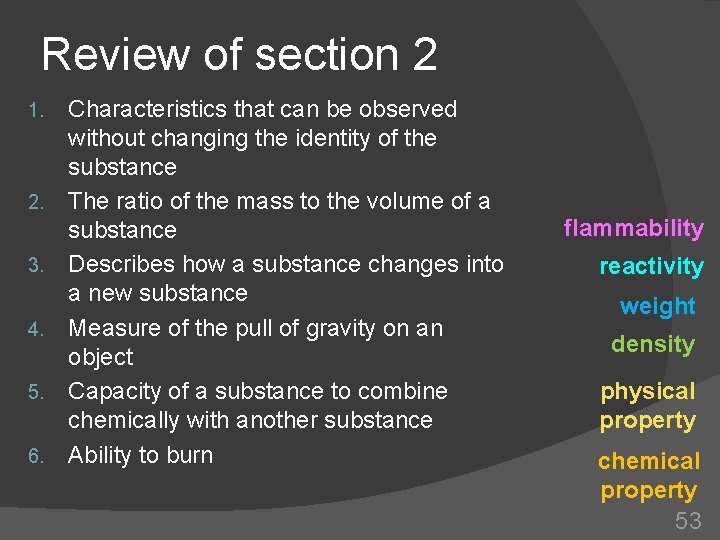
Review of section 2 1. 2. 3. 4. 5. 6. Characteristics that can be observed without changing the identity of the substance The ratio of the mass to the volume of a substance Describes how a substance changes into a new substance Measure of the pull of gravity on an object Capacity of a substance to combine chemically with another substance Ability to burn flammability reactivity weight density physical property chemical property 53

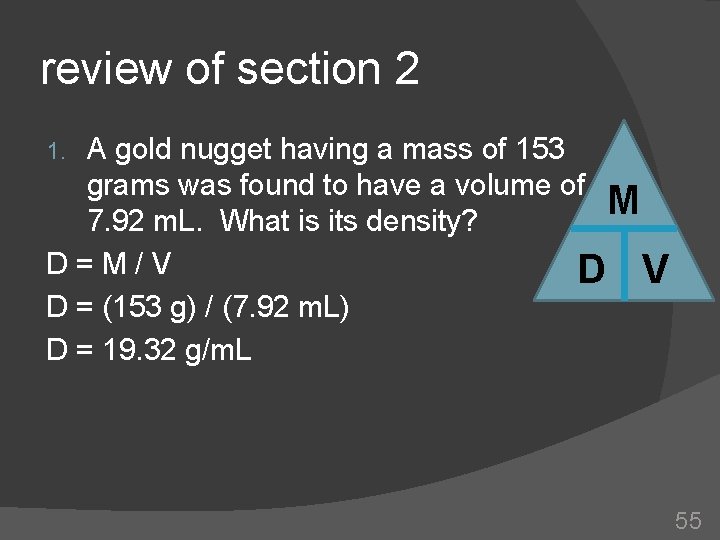
review of section 2 A gold nugget having a mass of 153 grams was found to have a volume of 7. 92 m. L. What is its density? 2. What is the mass of 4000 m. L of mercury if the density of mercury is 13. 6 g/m. L? 3. Calculate the volume of a sample of carbon tetrachloride having a mass of 82 grams and a density of 1. 60 g/m. L 1. 54

review of section 2 A gold nugget having a mass of 153 grams was found to have a volume of M 7. 92 m. L. What is its density? D=M/V D = (153 g) / (7. 92 m. L) D = 19. 32 g/m. L 1. 55

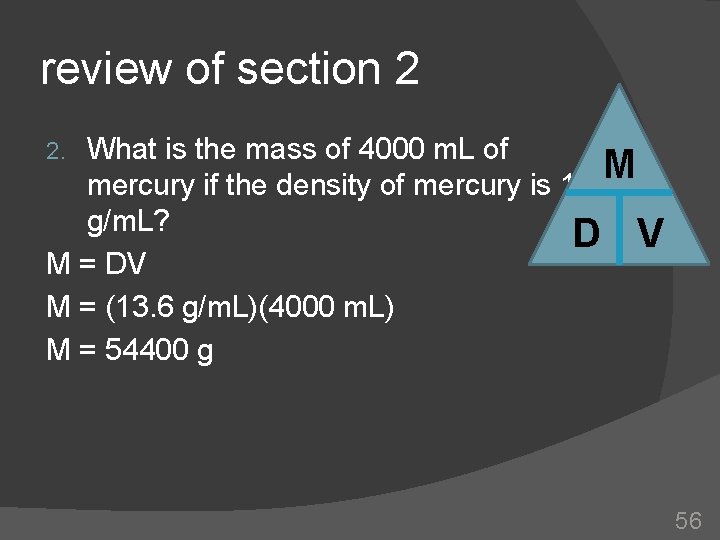
review of section 2 What is the mass of 4000 m. L of M mercury if the density of mercury is 13. 6 g/m. L? D V M = DV M = (13. 6 g/m. L)(4000 m. L) M = 54400 g 2. 56

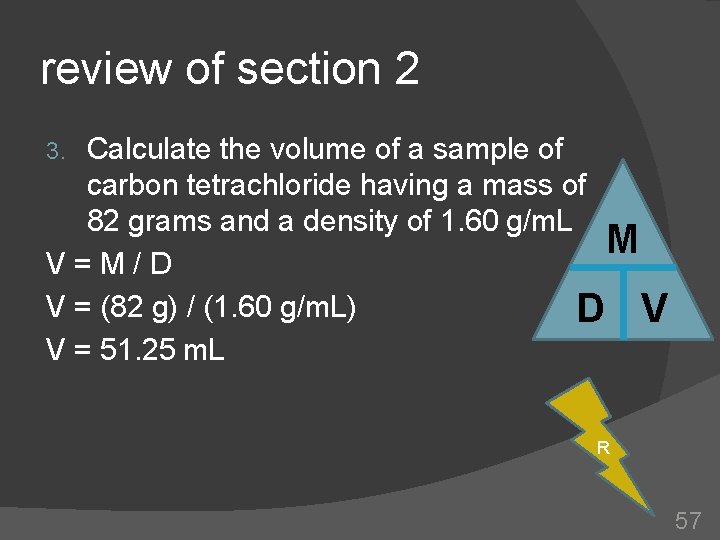
review of section 2 Calculate the volume of a sample of carbon tetrachloride having a mass of 82 grams and a density of 1. 60 g/m. L M V=M/D V = (82 g) / (1. 60 g/m. L) D V V = 51. 25 m. L 3. R 57

Section 3 CHANGES OF MATTER 58

12. Define and give an example of a physical change of matter. Physical change: A change of matter from one form to another without a change in chemical properties e. g. breaking chalk melting ice cutting hair crushing a can energy can be absorbed or released without the formation of a new material 59

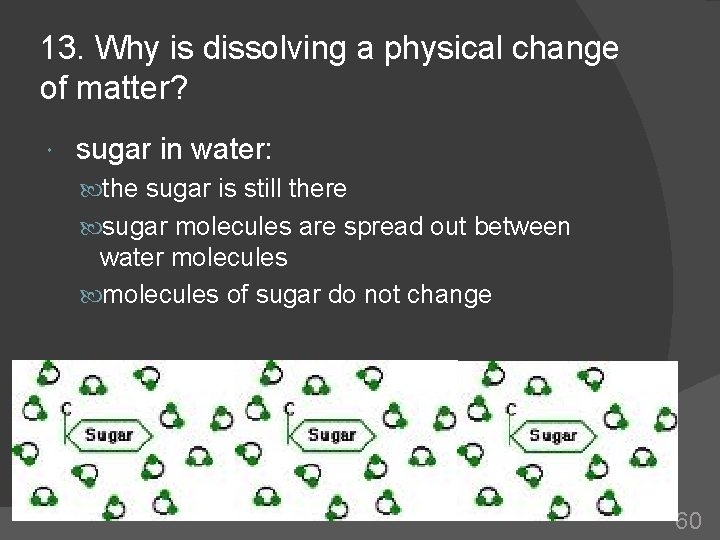
13. Why is dissolving a physical change of matter? sugar in water: the sugar is still there sugar molecules are spread out between water molecules of sugar do not change 60

14. Define and give an example of a chemical change of matter. chemical change a change that occurs when one or more substances change into entirely new substances with different properties examples leaves changing color in the fall effervescent tablets—release gas batteries die oxygen breathed in, carbon dioxide breathed out 61

chemical change examples chemical changes fruits & vegetables ripen food is digested baking burning food fading paint iron rusting chemical changes can only be reversed by other chemical changes 62

15. Why must mixtures and compounds be separated in different ways? mixtures can be separated by physical changes, they are NOT chemically combined compounds can only be broken down by chemical changes, they are chemically combined D 63

Section 3 Review Change of matter from one form to another without a change in chemical properties 2. Change of matter that occurs when one or more substances change into entirely new substance with different properties 1. physical change chemical change 64

Physical property or Chemical property? Color physical Magnetism physical Reactivity chemical Stability chemical Melting point physical Flammability chemical Ability to conduct physical heat

Physical property or Chemical property? Shape physical Strength physical texture physical Odor State of matter physical

Physical change or Chemical change? Fading of dye in cloth chemical Digestion of food chemical Baking a cake chemical Frying an egg chemical Burning coal chemical Kicking a football physical Crushing a can physical Cutting hair physical

Physical change or Chemical change? Growth of a plant chemical Melting butter physical Formation of clouds physical Tearing paper physical Exploding TNT chemical Melting of ice physical Dissolving sugar in water physical

Physical change or Chemical change? Burning butter chemical Healing of a wound chemical Perming your hair chemical Pounding a piece of copper physical Digging a hole in the ground physical Leaves turning color in the fall chemical

Chapter 2 Review pp. 70 73 Using Key Terms # 4 , 7 Understanding Ideas # 8 – 14 Critical Thinking # 20 , 21 Graphing Skills # 23 Math Skills # 24 – 26 Understanding Concepts # 1 – 3 Reading Skills # 7, 8 Interpreting Graphics # 9, 10 70