Chemistry Unit 2 Matter and its changes Chemistry
























- Slides: 45

Chemistry Unit 2: Matter and its changes

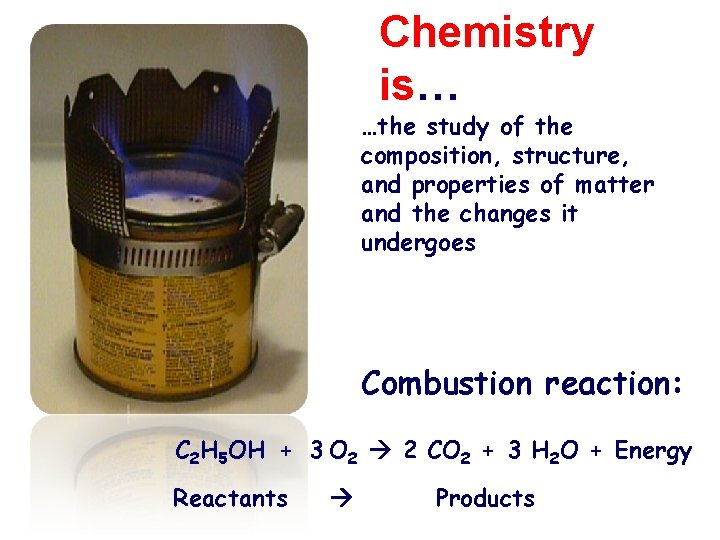
Chemistry is… …the study of the composition, structure, and properties of matter and the changes it undergoes Combustion reaction: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O + Energy Reactants Products

Matter Introductory Definitions matter: anything having mass and volume mass: the amount of matter in an object volume: the space an object occupies units: L, dm 3, m. L, cm 3 properties: describe the matter what it looks like, smells like; its mass, temp. , etc. how it behaves

Properties of Matter Extensive properties depend on the amount of matter that is present. Volume Length Height Mass Energy Content (think Calories!) Intensive properties do not depend on the amount of matter present. Melting point Boiling point Density Color Odor Conductivity Malleable Ductile

PHYSICAL PROPERTIES • characteristics that can be observed without changing the identity of the substance. • Examples: – – – mass volume color shape texture density

CHEMICAL PROPERTIES • describe the tendency of a substance to undergo chemical change(s), or transform into new substances • Examples: – flammability – reactivity

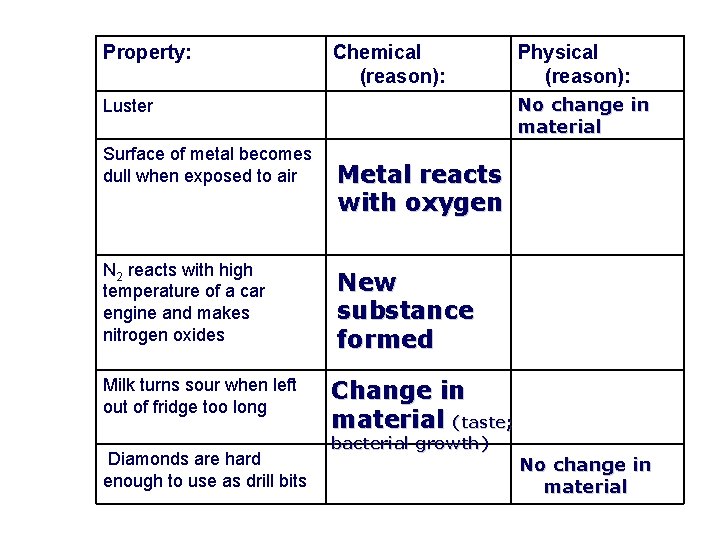
Property: Chemical (reason): No change in material Luster Surface of metal becomes dull when exposed to air N 2 reacts with high temperature of a car engine and makes nitrogen oxides Milk turns sour when left out of fridge too long Diamonds are hard enough to use as drill bits Physical (reason): Metal reacts with oxygen New substance formed Change in material (taste; bacterial growth) No change in material

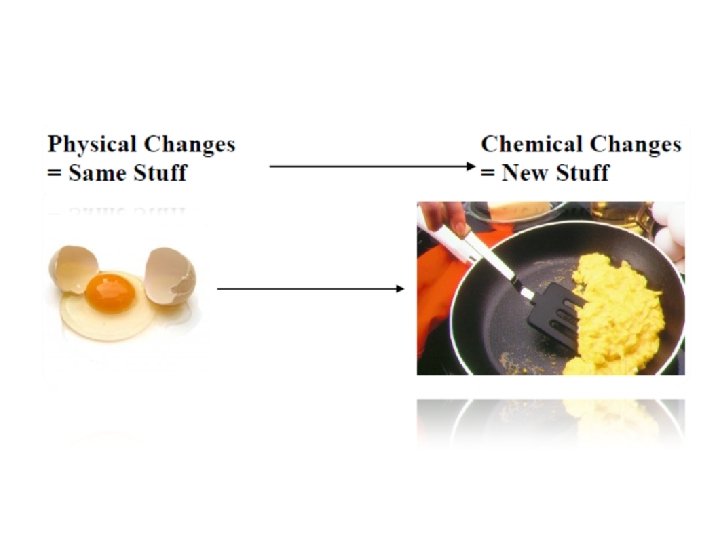
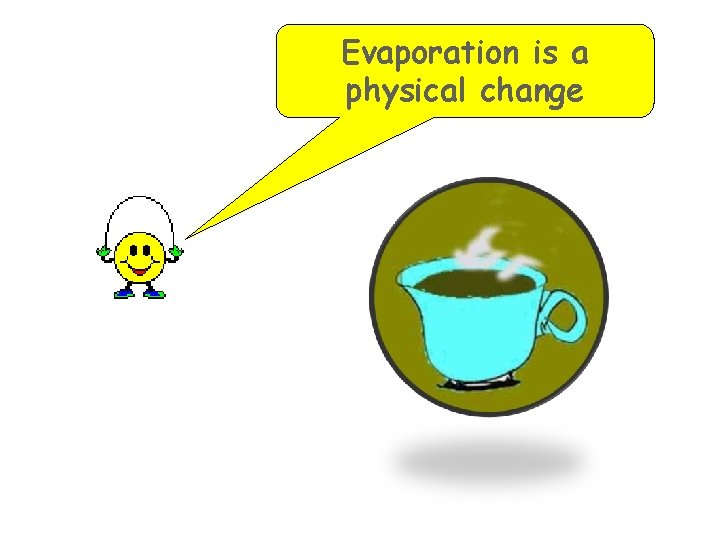
Physical Change A change in a substance that does not involve a change in the identity of the substance. Example: Phase Changes (liquid to gas) Evaporation allows the solvent to be removed from the solute by boiling.

Chemical Change A change in which one or more substances are converted into different substances. Heat and light are often evidence of a chemical change.

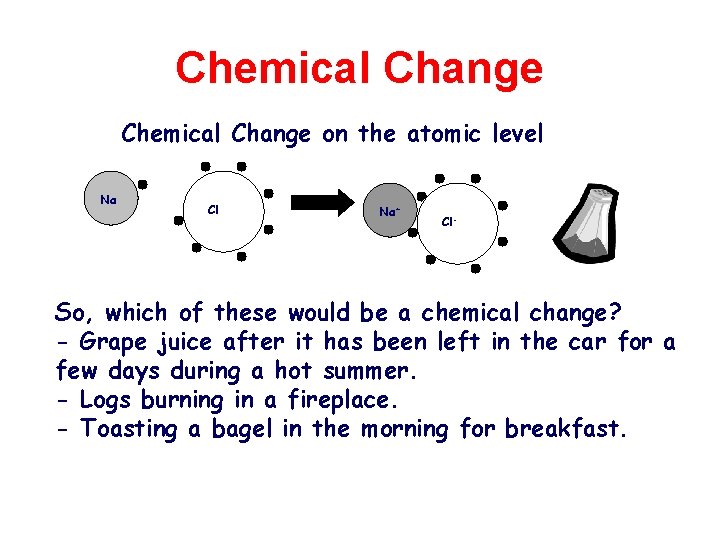
Chemical Change on the atomic level Na Cl Na+ Cl- So, which of these would be a chemical change? - Grape juice after it has been left in the car for a few days during a hot summer. - Logs burning in a fireplace. - Toasting a bagel in the morning for breakfast.

Evidence of Chemical Change Key to a chemical reaction is the formation of a new substance *Evidence of new substance (s): – gas is produced – new taste, smell, or sound is produced – light and/or electricity is produced color changes temperature changes – Precipitate forms • a solid that forms from a solution during a chemical reaction.


Evaporation is a physical change

Breaking is a physical change.

Boiling is a change of state, and therefore a physical change!

Rusting is a Chemical Change

Burning is a Chemical Change

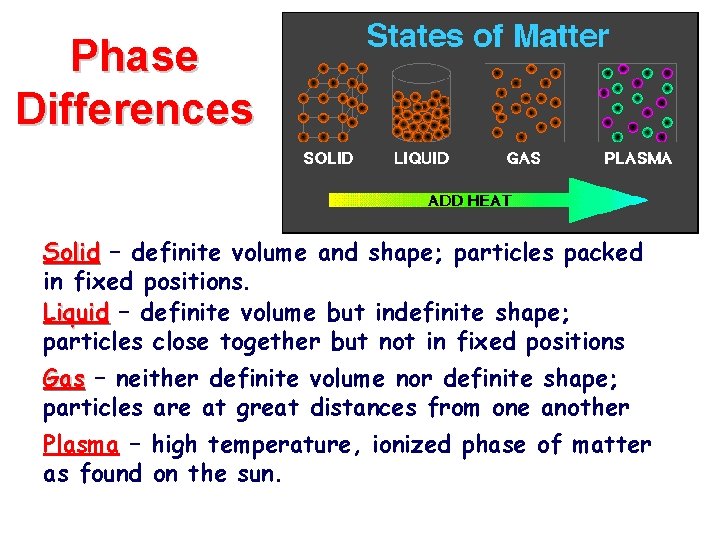
Phase Differences Solid – definite volume and shape; particles packed in fixed positions. Liquid – definite volume but indefinite shape; particles close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun.

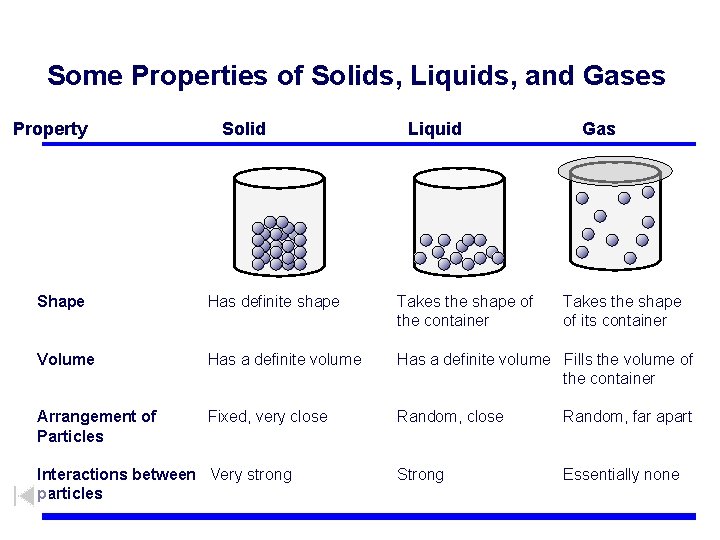
Some Properties of Solids, Liquids, and Gases Property Solid Liquid Gas Shape Has definite shape Takes the shape of the container Volume Has a definite volume Fills the volume of the container Arrangement of Particles Fixed, very close Random, far apart Strong Essentially none Interactions between Very strong particles Takes the shape of its container

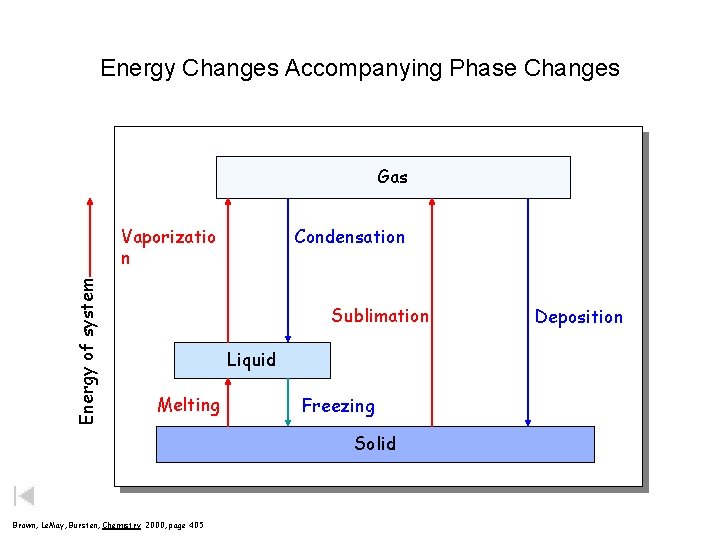
Energy Changes Accompanying Phase Changes Gas Energy of system Vaporizatio n Condensation Sublimation Liquid Melting Freezing Solid Brown, Le. May, Bursten, Chemistry 2000, page 405 Deposition

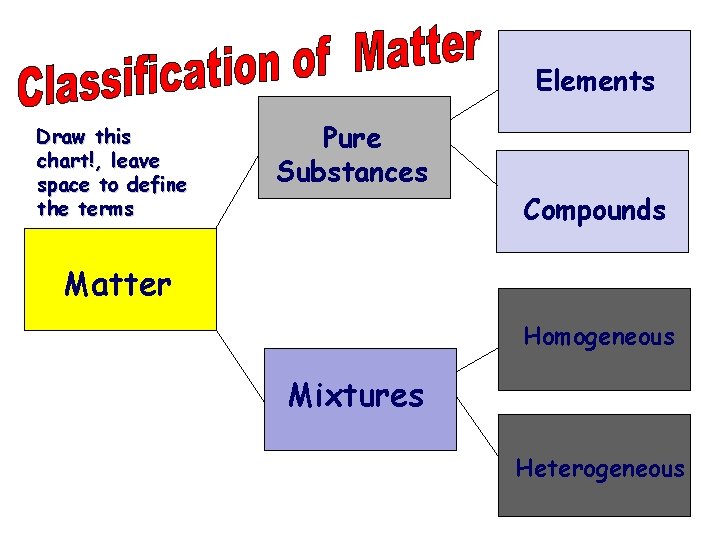
Elements Draw this chart!, leave space to define the terms Pure Substances Compounds Matter Homogeneous Mixtures Heterogeneous

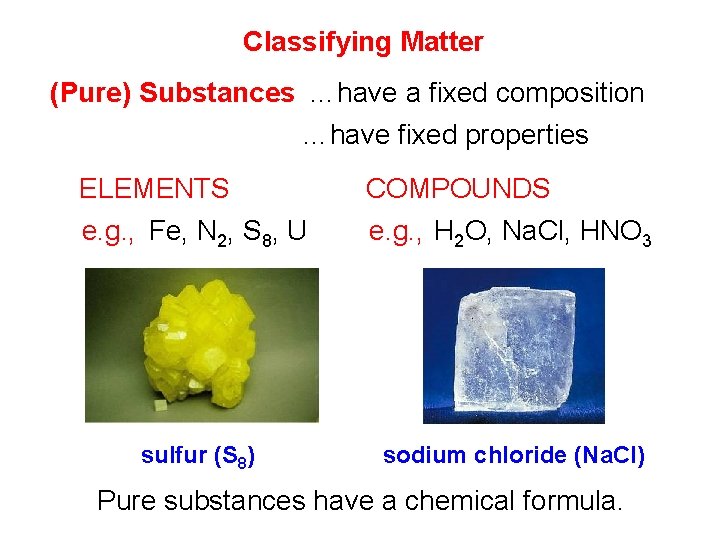
Classifying Matter (Pure) Substances …have a fixed composition …have fixed properties ELEMENTS COMPOUNDS e. g. , Fe, N 2, S 8, U e. g. , H 2 O, Na. Cl, HNO 3 sulfur (S 8) sodium chloride (Na. Cl) Pure substances have a chemical formula.

Mixtures two or more substances mixed together …have varying composition …have varying properties The substances are NOT chemically bonded, and they… retain their individual properties. Tea, orange juice, oceans, and air are mixtures.

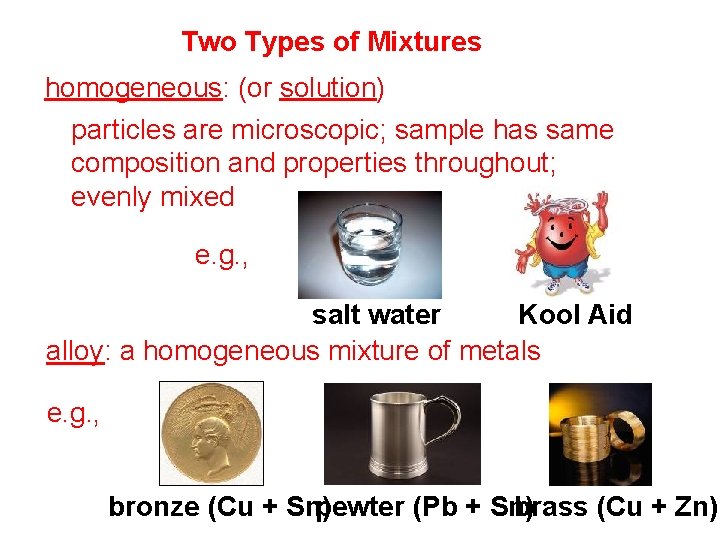
Two Types of Mixtures homogeneous: (or solution) particles are microscopic; sample has same composition and properties throughout; evenly mixed e. g. , salt water Kool Aid alloy: a homogeneous mixture of metals e. g. , bronze (Cu + Sn) pewter (Pb + Sn) brass (Cu + Zn)

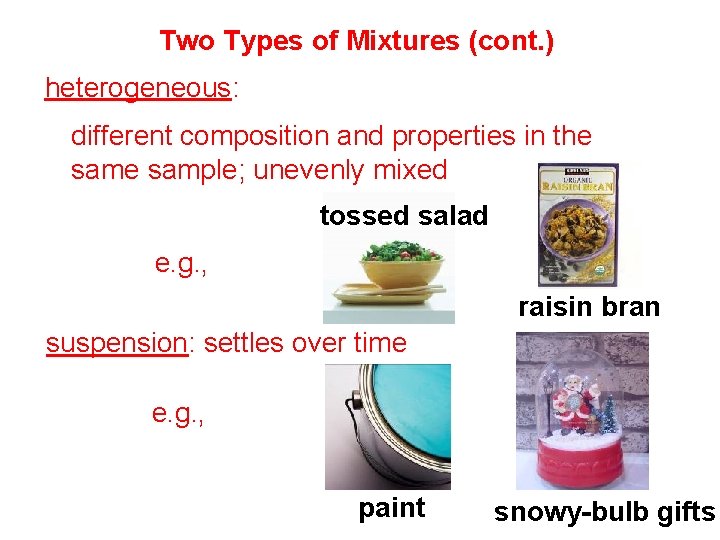
Two Types of Mixtures (cont. ) heterogeneous: different composition and properties in the sample; unevenly mixed tossed salad e. g. , raisin bran suspension: settles over time e. g. , paint snowy-bulb gifts

EXAMPLES • What type of matter are each of the following… ?

SAND

SAND Heterogeneous mixture

Salt (Na. Cl)

Salt (Na. Cl) COMPOUND

Air

Air • Homogeneous mixture of: Many gases make up mixture, but it looks like it is all one gas. Nitrogen, N 2 78. 08% Oxygen, O 2 20. 95% Argon, Ar 0. 93% Carbon dioxide, CO 2 0. 033% Neon, Ne 0. 0018% Helium, He 0. 00052% Methane, CH 4 0. 0002% Krypton, Kr 0. 00011% Nitrogen(I) oxide, N 2 O 0. 00005% Hydrogen, H 2 0. 00005% Xenon, Xe 0. 0000087% Ozone, O 3 0. 000001%

Gold

Gold ELEMENT: Au

Bronze

Bronze Homogeneous mixture of copper and tin (alloy: mixture of metals)

Salad Dressing

Salad Dressing: Heterogeneous

Separation of a Mixture The constituents of the mixture retain their identity and may be separated by physical means.

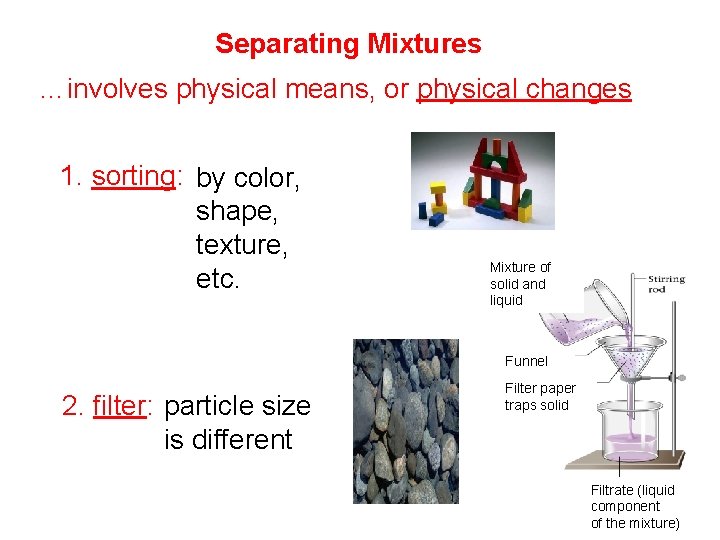
Separating Mixtures …involves physical means, or physical changes 1. sorting: by color, shape, texture, etc. Mixture of solid and liquid Funnel 2. filter: particle size is different Filter paper traps solid Filtrate (liquid component of the mixture)

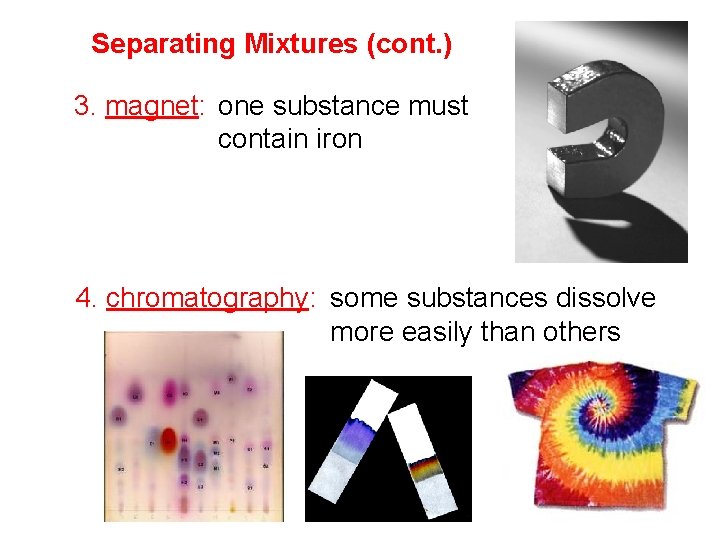
Separating Mixtures (cont. ) 3. magnet: one substance must contain iron 4. chromatography: some substances dissolve more easily than others

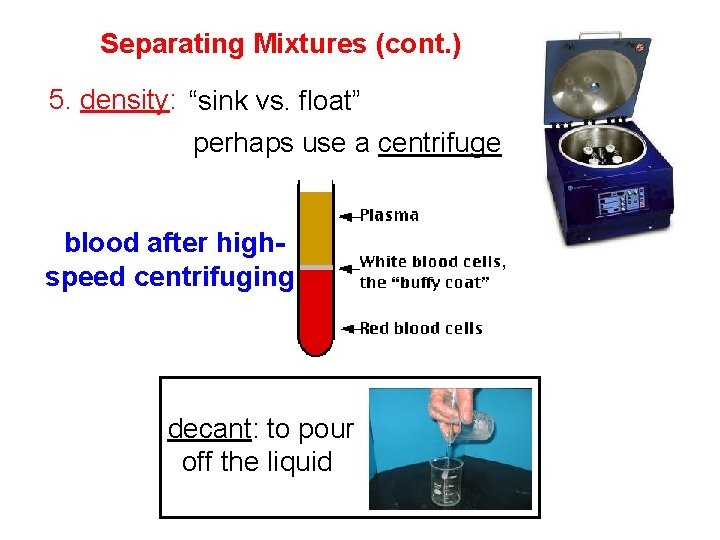
Separating Mixtures (cont. ) 5. density: “sink vs. float” perhaps use a centrifuge blood after highspeed centrifuging decant: to pour off the liquid

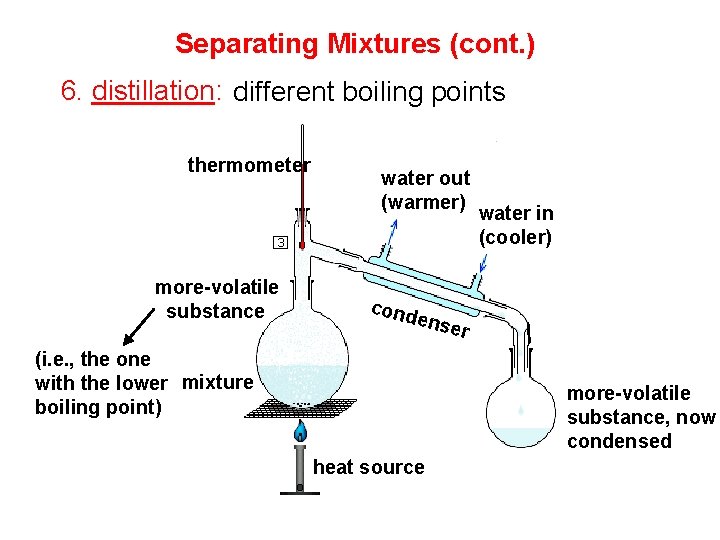
Separating Mixtures (cont. ) 6. distillation: different boiling points thermometer more-volatile substance water out (warmer) cond ense (i. e. , the one with the lower mixture boiling point) water in (cooler) r more-volatile substance, now condensed heat source

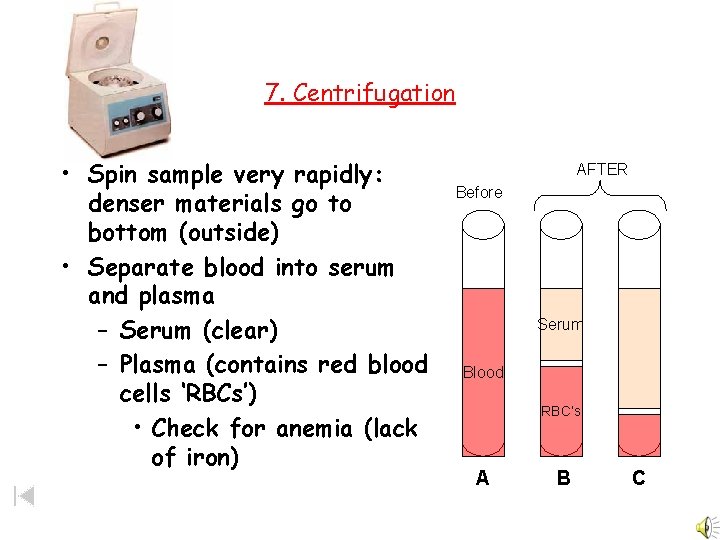
7. Centrifugation • Spin sample very rapidly: denser materials go to bottom (outside) • Separate blood into serum and plasma – Serum (clear) – Plasma (contains red blood cells ‘RBCs’) • Check for anemia (lack of iron) AFTER Before Serum Blood RBC’s A B C

No chemical reactions are needed to separate mixtures; substances are NOT bonded. dental amalgam