CHANGES OF STATES OF MATTER Phase Changes Matter






- Slides: 18

CHANGES OF STATES OF MATTER Phase Changes

Matter = has mass & takes up space (has volume) Volume = amount of space an object takes up

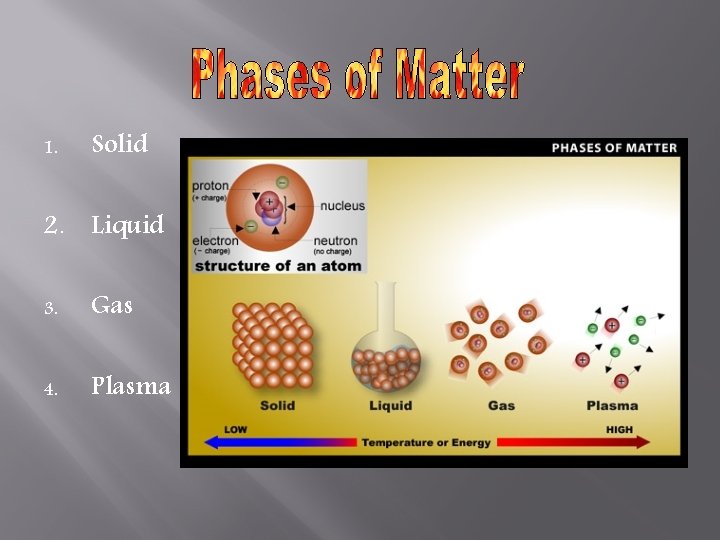
1. Solid 2. Liquid 3. Gas 4. Plasma

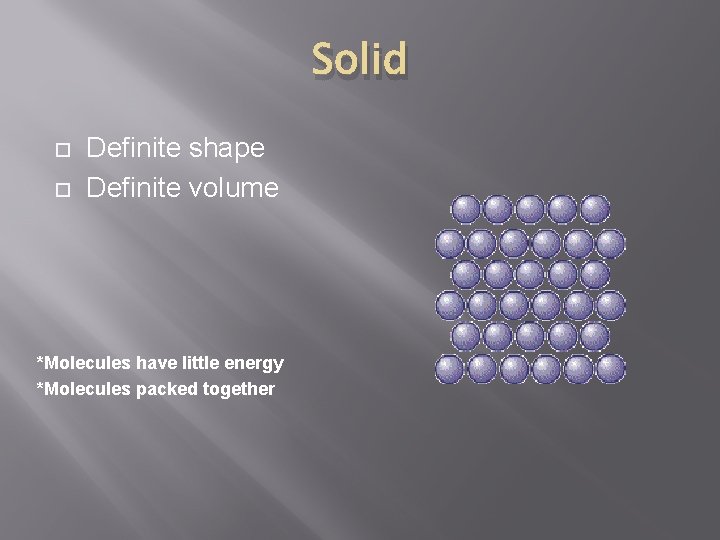
Solid Definite shape Definite volume *Molecules have little energy *Molecules packed together

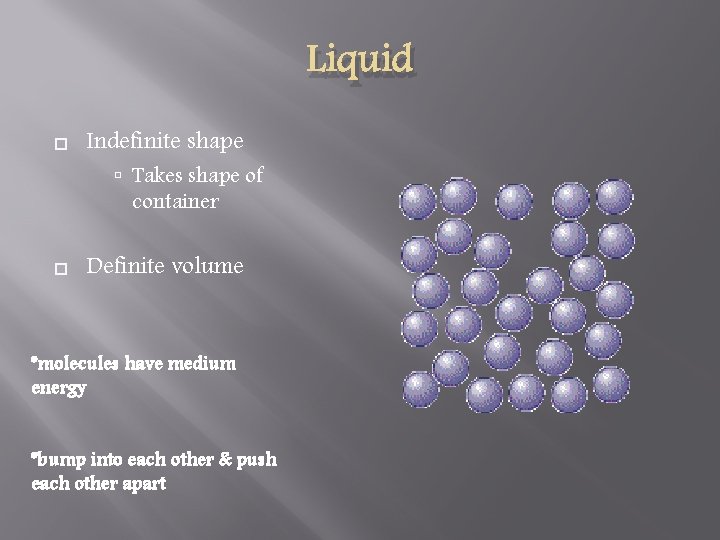
Liquid Indefinite shape Takes shape of container Definite volume *molecules have medium energy *bump into each other & push each other apart

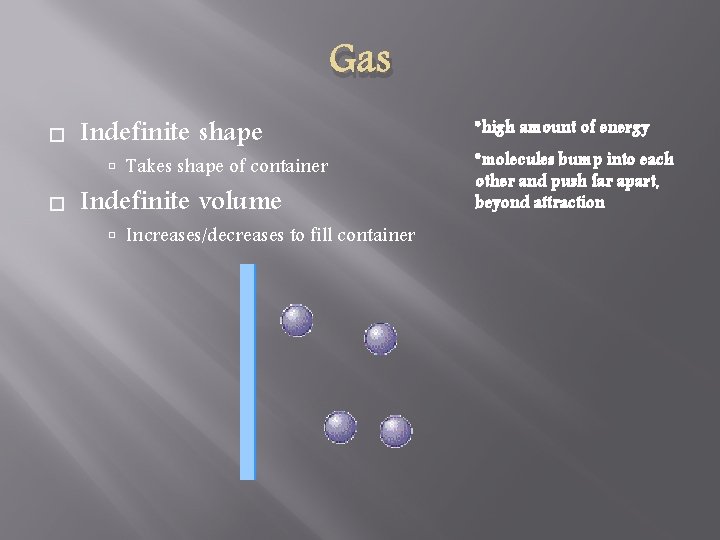
Gas Indefinite shape Takes shape of container Indefinite volume Increases/decreases to fill container *high amount of energy *molecules bump into each other and push far apart, beyond attraction


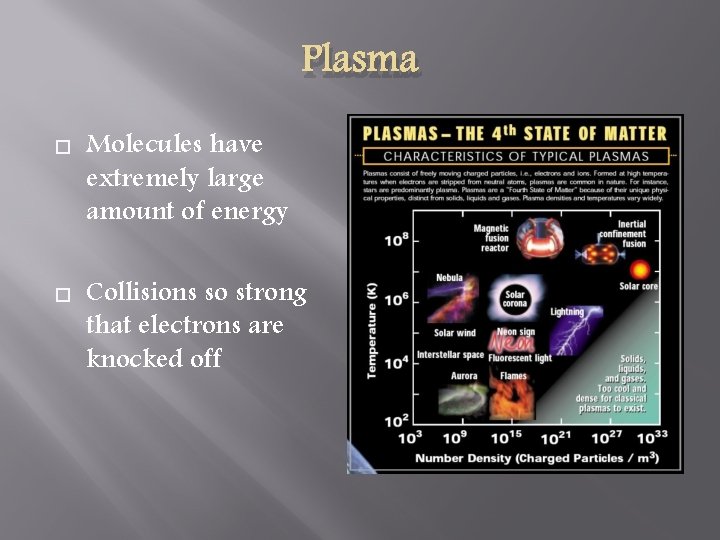
Plasma Molecules have extremely large amount of energy Collisions so strong that electrons are knocked off


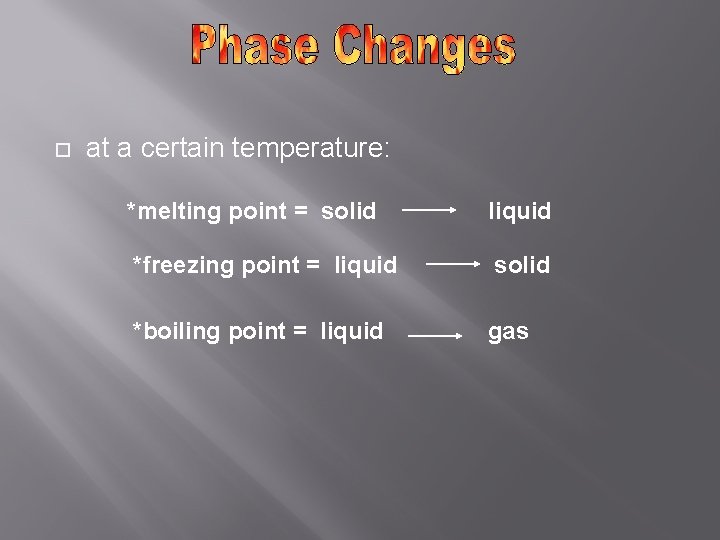
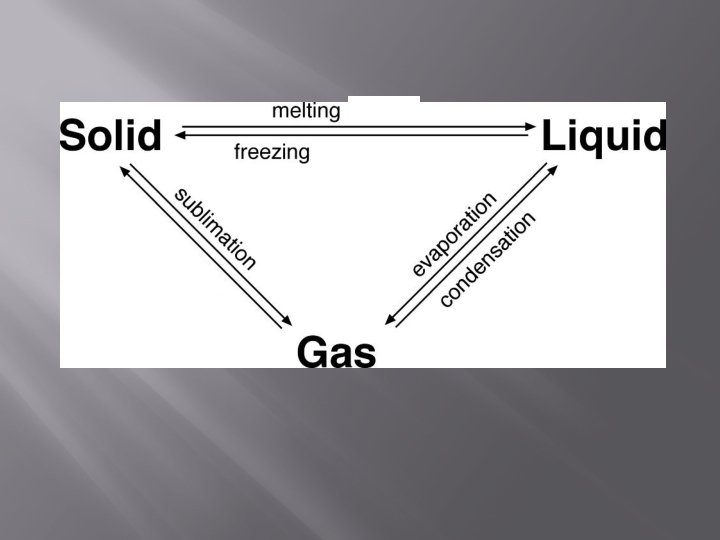
at a certain temperature: *melting point = solid liquid *freezing point = liquid solid *boiling point = liquid gas

How does the physical state of matter change? All Matter has thermal energy Increase thermal energy – molecules move faster Decrease thermal energy – molecules move slower

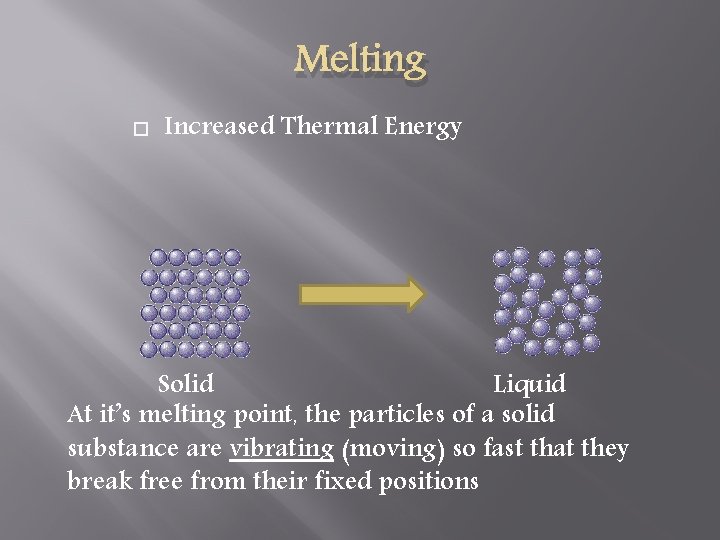
Melting Increased Thermal Energy Solid Liquid At it’s melting point, the particles of a solid substance are vibrating (moving) so fast that they break free from their fixed positions

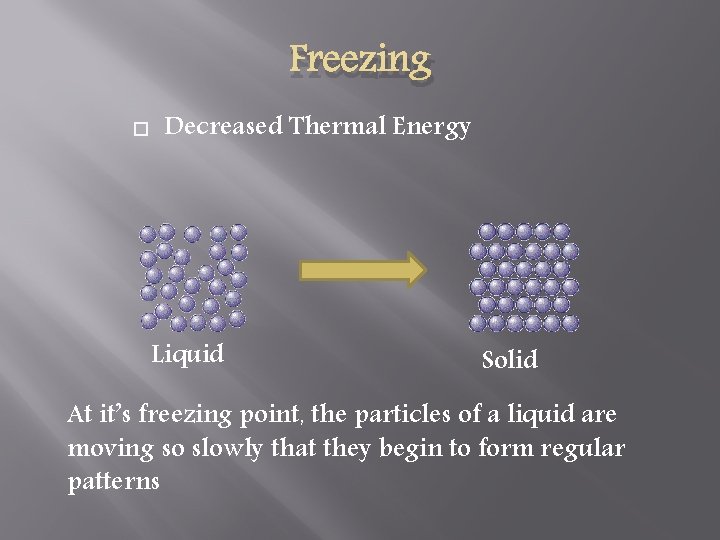
Freezing Decreased Thermal Energy Liquid Solid At it’s freezing point, the particles of a liquid are moving so slowly that they begin to form regular patterns

Condensation Decreased Thermal Energy Gas Liquid Condensation occurs when particles in a gas loose enough thermal energy (slow down) to form a liquid (ex. Formation of a cloud)

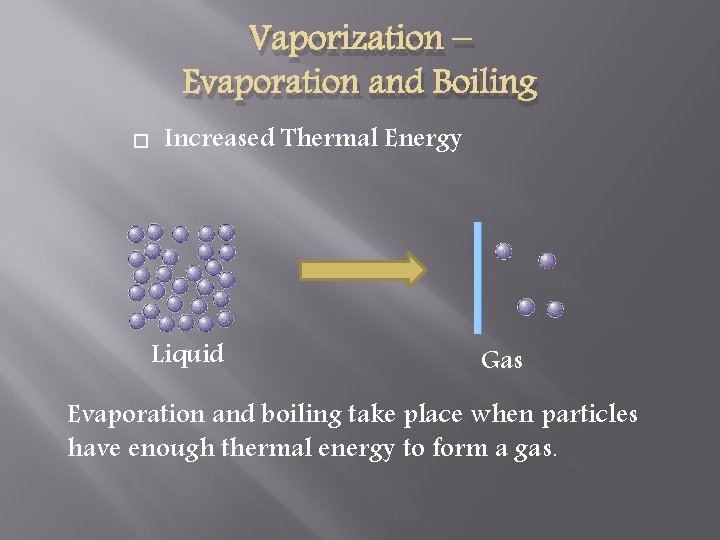
Vaporization – Evaporation and Boiling Increased Thermal Energy Liquid Gas Evaporation and boiling take place when particles have enough thermal energy to form a gas.

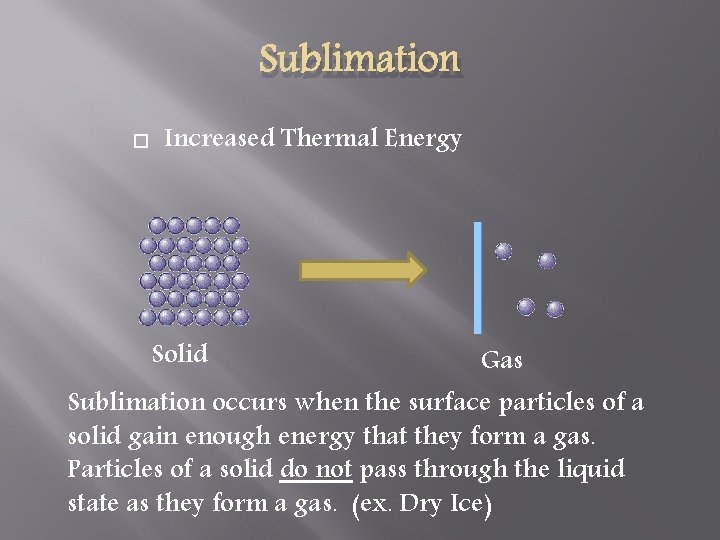
Sublimation Increased Thermal Energy Solid Gas Sublimation occurs when the surface particles of a solid gain enough energy that they form a gas. Particles of a solid do not pass through the liquid state as they form a gas. (ex. Dry Ice)

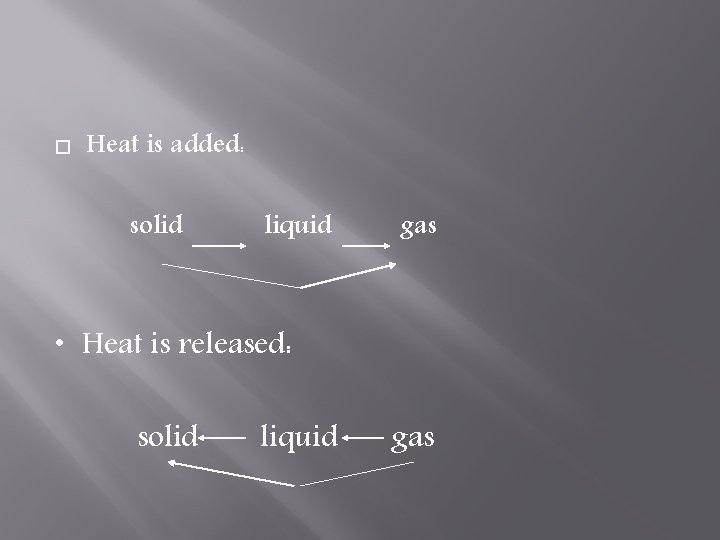
Heat is added: solid liquid gas • Heat is released: solid liquid gas

 6 common phase changes
6 common phase changes Phase changes of matter
Phase changes of matter Mobile phase and stationary phase
Mobile phase and stationary phase Hplc detector types
Hplc detector types Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Phase to phase voltage
Phase to phase voltage Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Csce 441
Csce 441 Tswett pronunciation
Tswett pronunciation Line vs phase voltage
Line vs phase voltage Examples of phase change
Examples of phase change Which of these phase changes is an endothermic process?
Which of these phase changes is an endothermic process? Heating cooling curve
Heating cooling curve Phase changes
Phase changes 6 phase changes
6 phase changes Are phase changes reversible
Are phase changes reversible Phase change worksheet answers
Phase change worksheet answers Elizabeth mulroney
Elizabeth mulroney