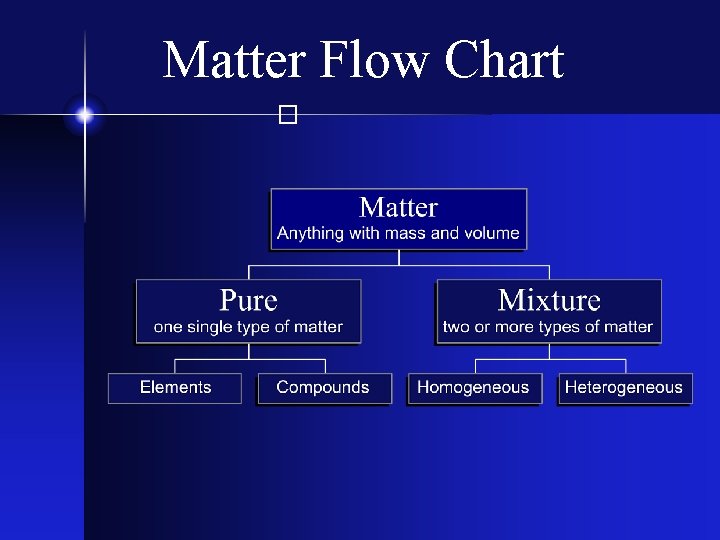
Types of Matter The Matter Flow Chart Matter









- Slides: 27

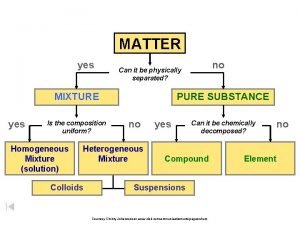
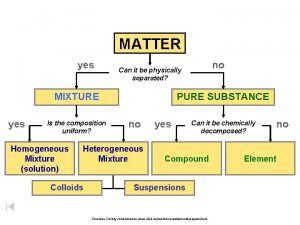
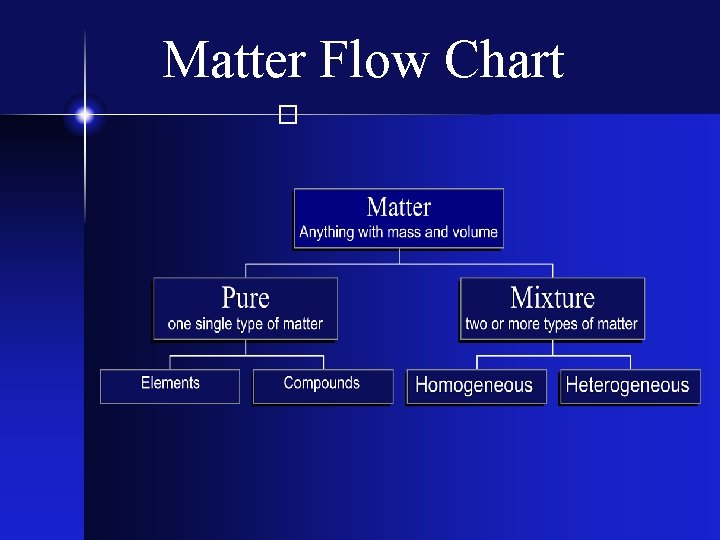
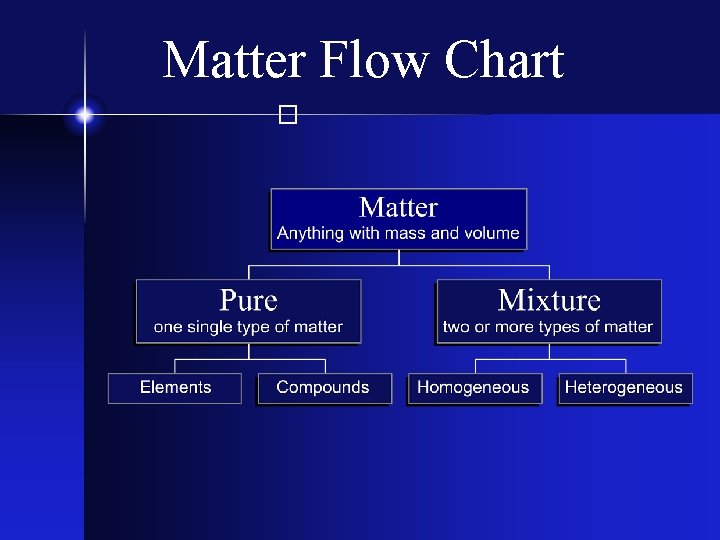
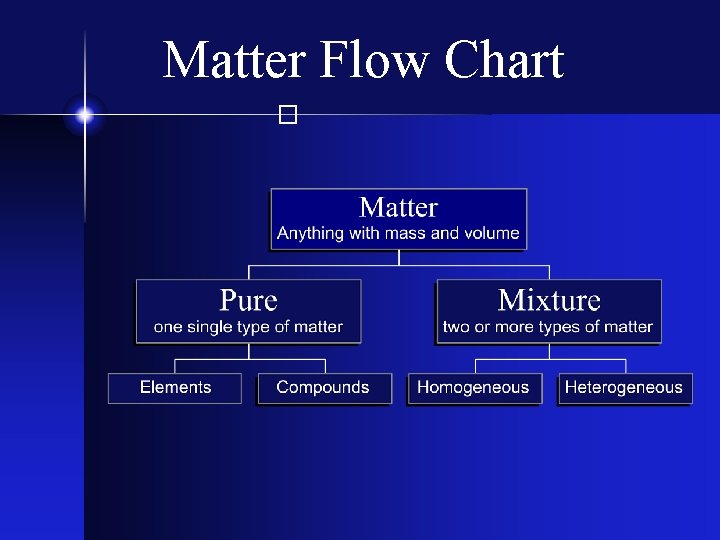
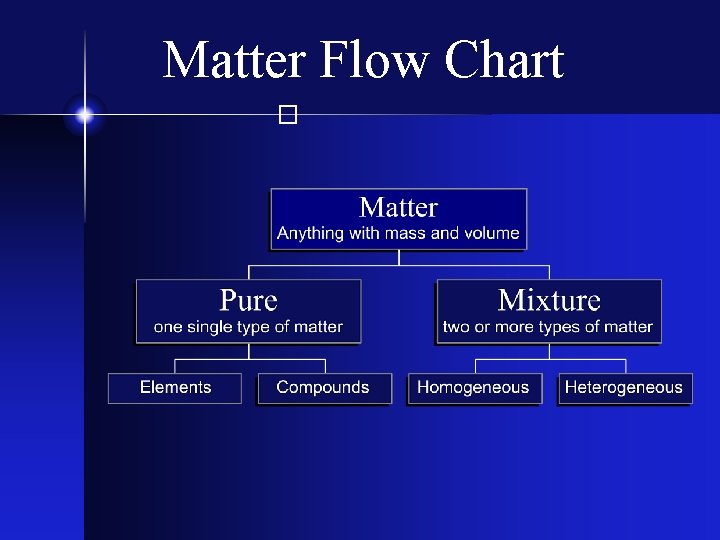
Types of Matter � The Matter Flow Chart

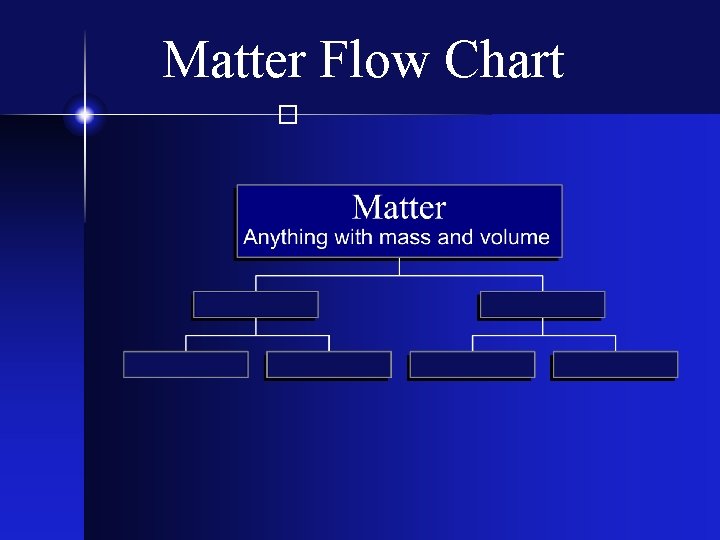
Matter Flow Chart �

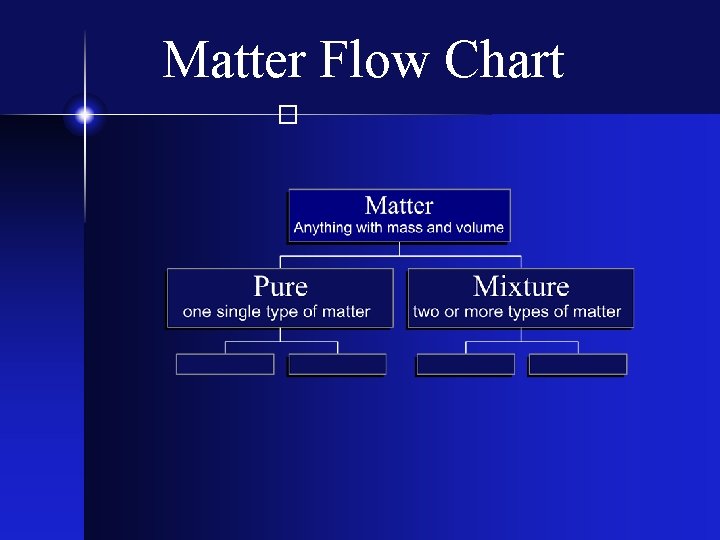
Matter Flow Chart �

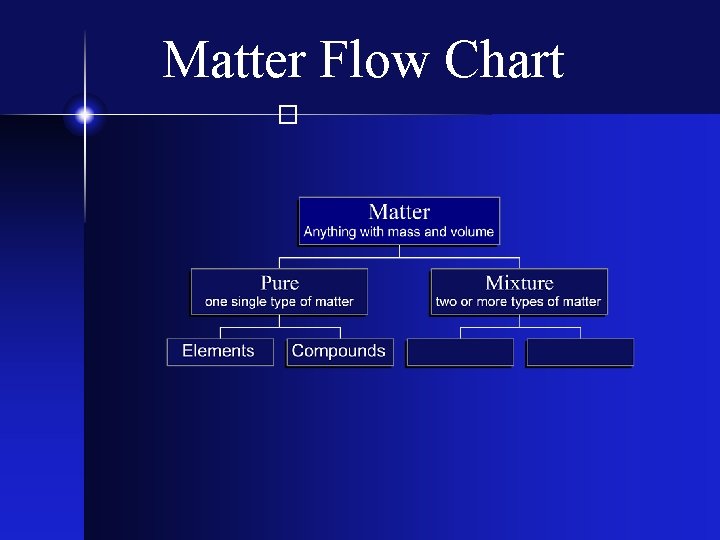
Matter Flow Chart �

Matter Flow Chart �

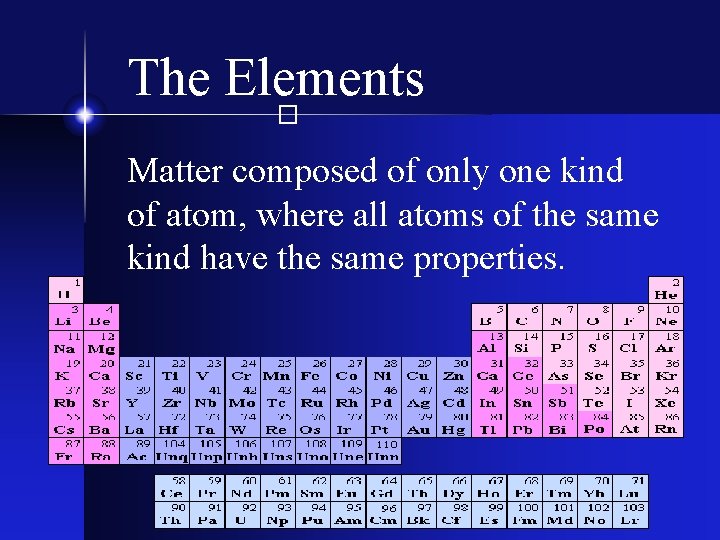
The Elements � Matter composed of only one kind of atom, where all atoms of the same kind have the same properties.

The Elements � § 90 different types exist naturally on Earth § Almost all known matter is composed of them § Common examples: H He O 2 Na Cl Fe Au sodium chlorine

The Elements � § An elemental substance can not be broken down by chemical processes § An elemental substance can not be converted into a different element by chemical processes “allotropes” of pure carbon graphite diamond charcoal

Matter Flow Chart �

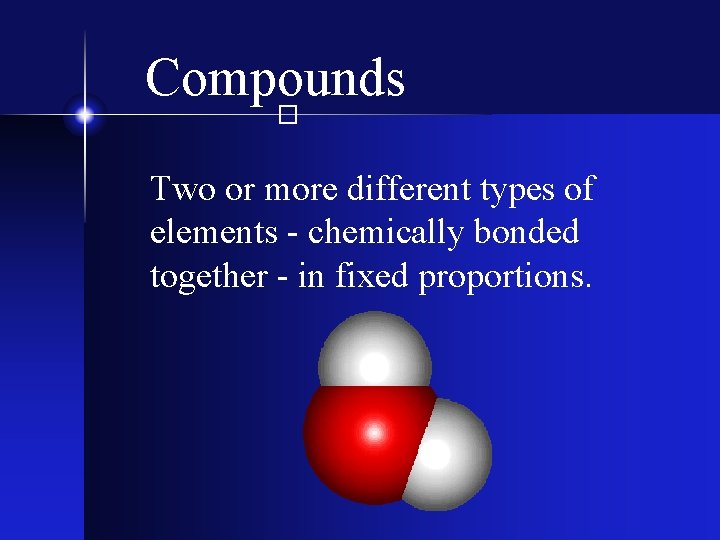
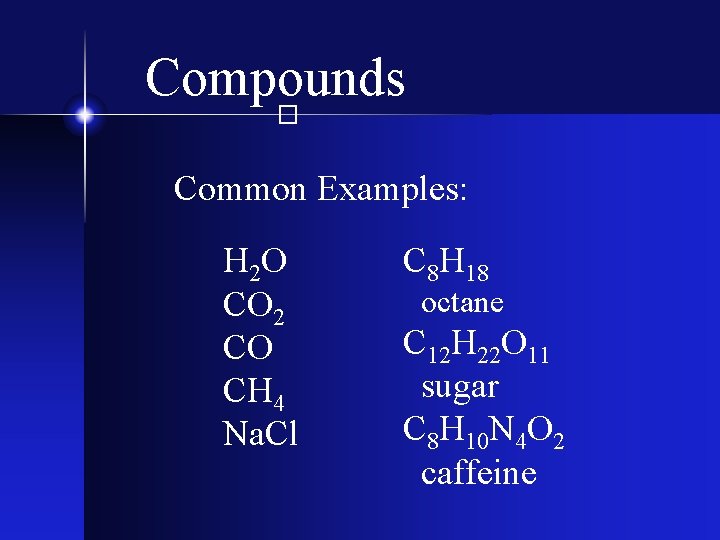
Compounds � Two or more different types of elements - chemically bonded together - in fixed proportions.

Compounds � Common Examples: H 2 O CO 2 CO CH 4 Na. Cl C 8 H 18 octane C 12 H 22 O 11 sugar C 8 H 10 N 4 O 2 caffeine

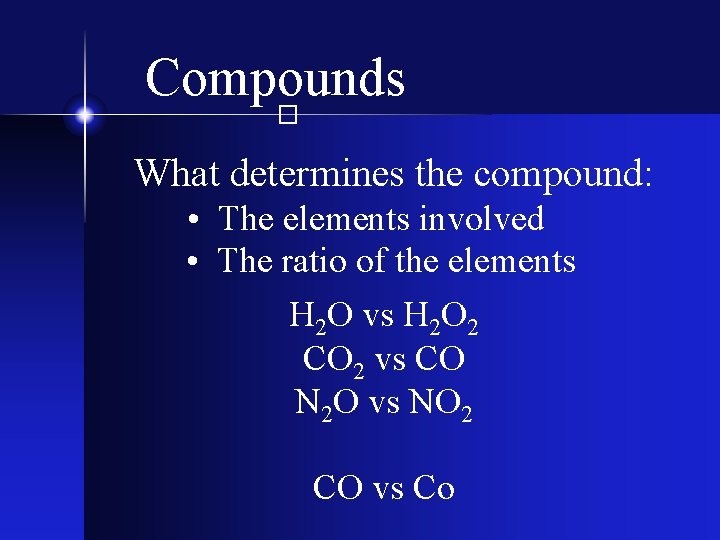
Compounds � What determines the compound: • The elements involved • The ratio of the elements H 2 O vs H 2 O 2 CO 2 vs CO N 2 O vs NO 2 CO vs Co

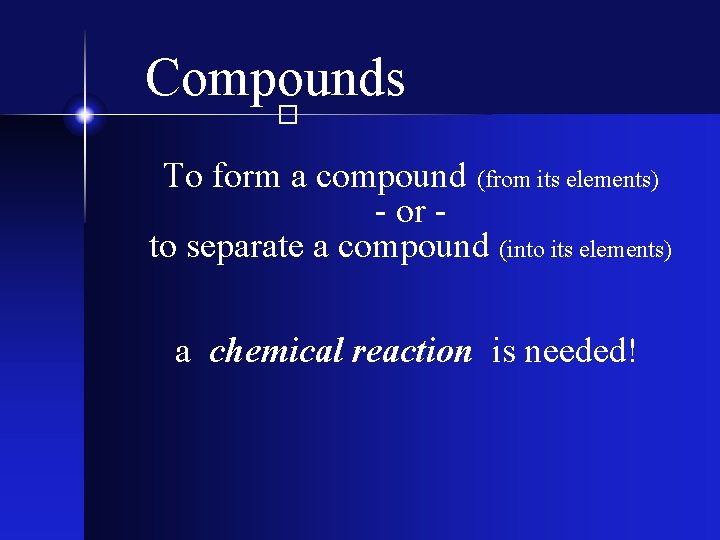
Compounds � To form a compound (from its elements) - or to separate a compound (into its elements) a chemical reaction is needed!

Matter Flow Chart �

Homogeneous Mixtures � A mixture that is uniform in its properties throughout any given sample. It is completely mixed! “Solutions”

Homogeneous Mixtures � Examples: Solids Alloys - metallic mixtures • Brass (Cu and Zn) • Bronze (Cu and Sn) • Steel (Fe, C, etc. ) • Pewter (97% Sn, Cu, Bi) (Used to be Pb and Sn)

Homogeneous Mixtures � Examples: Liquids • salt water • tea • sugar water • pop • apple juice The liquid must be “clearly transparent”

Homogeneous Mixtures � Examples: Gases • Air (N 2 and O 2, and others) • “Trimix” (N 2, He and O 2 for deep scuba) • Any combination of gases will mix thoroughly

Homogeneous Mixtures � Separated by physical processes: Examples: Evaporation

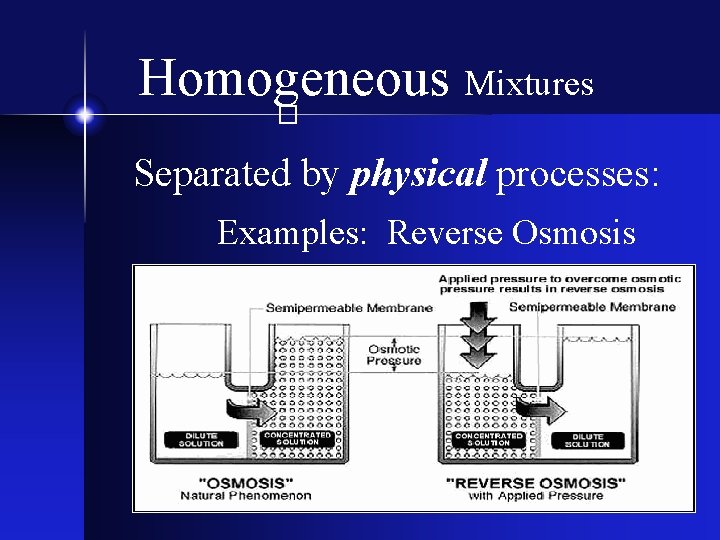
Homogeneous Mixtures � Separated by physical processes: Examples: Reverse Osmosis

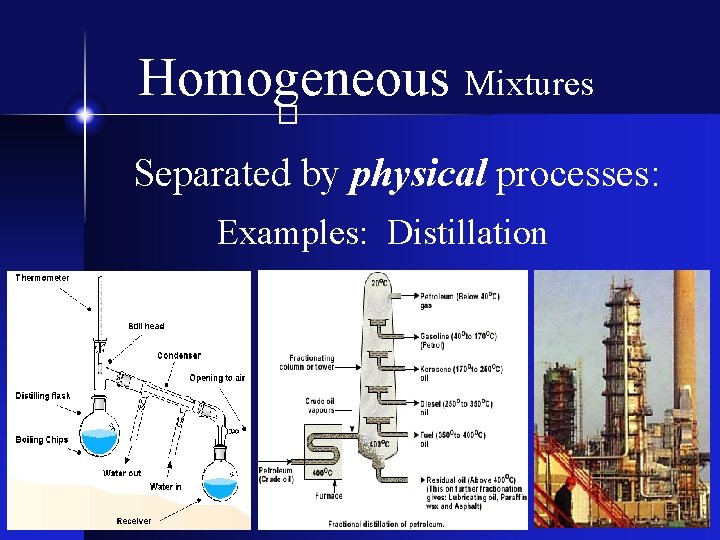
Homogeneous Mixtures � Separated by physical processes: Examples: Distillation

Matter Flow Chart �

Heterogeneous Mixtures � A mixture that consists of physically distinct parts, each with different properties. Not mixed very well! “Chunky”

Heterogeneous Mixtures � Examples: • Granite • Rocky Road Ice Cream • Soil • Lasagna • Oatmeal

Heterogeneous Mixtures � Also separated by physical processes: Examples: • Filtration • Floatation • Also, any method that works on homogeneous mixtures, would also work on heterogeneous mixtures

Matter Flow Chart �

1) 2) 3) 4) 5) Element Compound Homogeneous Heterogeneous � A) B) C) D) An apple Bronze Copper Na. Cl O 2 6) Nitrogen 7) Sand 8) Pure water 9) Tap water 10) Orange juice
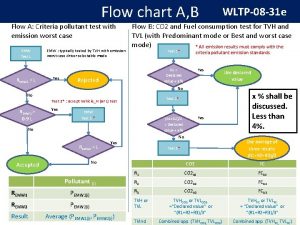
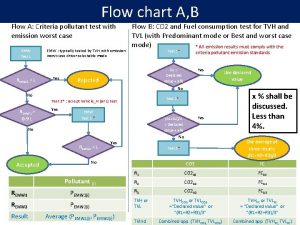
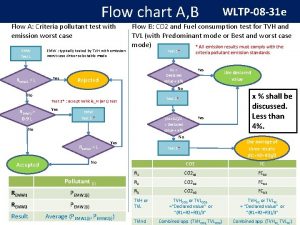
 Flow chart of matter
Flow chart of matter Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Oikos meaning
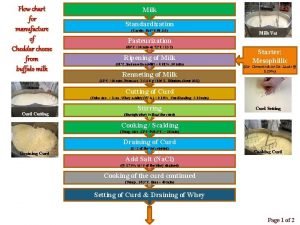
Oikos meaning Cheddar cheese production flow chart
Cheddar cheese production flow chart Matter flowchart
Matter flowchart Homogeneous mixture vs compound
Homogeneous mixture vs compound Classification of matter flow chart
Classification of matter flow chart Is the composition uniform?
Is the composition uniform? Composition of matter flow chart
Composition of matter flow chart Matter flowchart
Matter flowchart Classification of matter flow chart
Classification of matter flow chart Mixture flow chart
Mixture flow chart What is a heterogeneous mixture that never settles
What is a heterogeneous mixture that never settles Immunity definition
Immunity definition Matter table
Matter table Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân