Changes in Matter MATTER PHYSICAL PROPERTIES PHYSICAL CHANGES













- Slides: 16

Changes in Matter MATTER PHYSICAL PROPERTIES & PHYSICAL CHANGES CHEMICAL PROPERTIES & CHEMICAL CHANGES

VOCABULARY • matter • mass • volume • density • solid • solubility • liquid • chemical property • gas • Chemical change • physical property • Physical change • Solute • solvent

WHAT IS MATTER? • • Matter is anything that has mass and takes up space. Matter can have both physical and chemical properties.

STATES OF MATTER • • Volume is the amount of space a sample of matter occupies. A solid is a state of matter with a definite shape and volume. A liquid is a state of matter with a definite volume but not a definite shape. A gas is a state of matter without a definite shape or a definite volume.

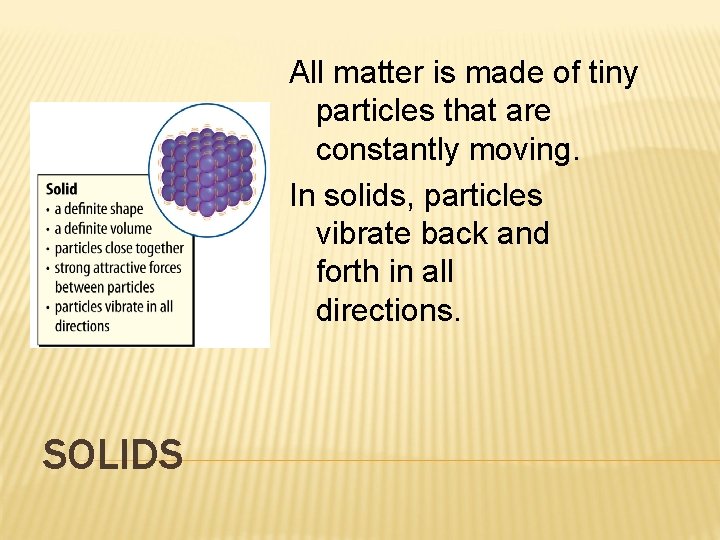
All matter is made of tiny particles that are constantly moving. In solids, particles vibrate back and forth in all directions. SOLIDS

In liquids, the distance between particles is greater and they can slide past one another. LIQUIDS

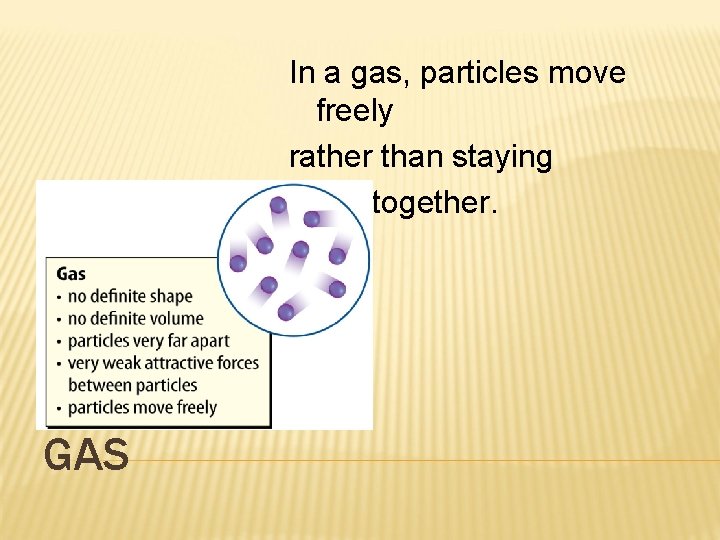
In a gas, particles move freely rather than staying close together. GAS

PHYSICAL PROPERTY: • Any characteristic of matter that you can observe without changing the identity of the substances that make it up EXAMPLES OF PHYSICAL PROPERTIES: • • • • State of matter Temperature Color Size Odor Density Volume Malleability Ductility Solubility Weight Melting point Boiling point WHAT IS A PHYSICAL PROPERTY?

WHAT IS A PHYSICAL CHANGE? • • • Physical change: A change in the size, shape, form, or state of matter that does not change the matter’s identity Matter can change in many physical and chemical ways. When a physical change occurs, the chemical properties of the matter stay the same.

PHYSICAL CHANGES CONT…. • • • Changes in the state of matter are physical changes. Melting and boiling are both changes in state. Changes in energy cause changes in the state of matter.

CHEMICAL PROPERTIES Chemical Properties describe a substance based on the ability to change into a new substance with different properties Example: A piece of wood being burned will create new substances of ash and smoke Wood has the chemical property of flammability- the ability to burn

COMMON CHEMICAL PROPERTIES… Flammability Non flammability Reactivity with oxygen Reactivity with acid Reactivity with water ** Reactivity simply means that when 2 substances get together, something can happen!

REMEMBER!!! **You can observe chemical properties ONLY in substances in which the identity of the substance could change

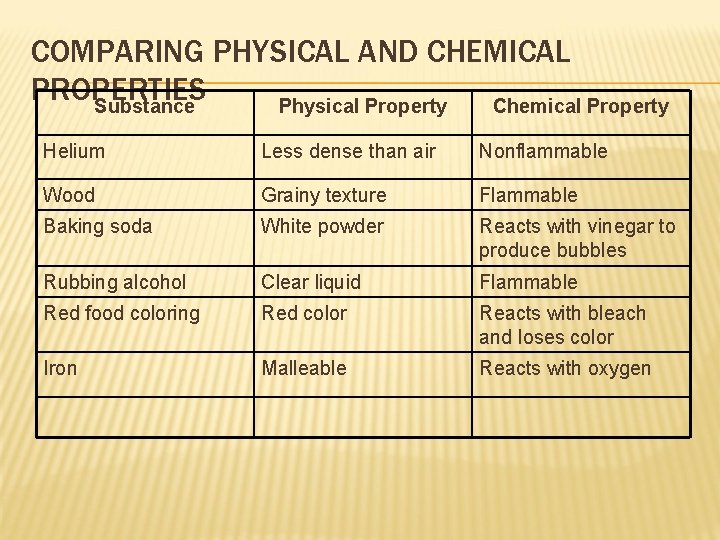
COMPARING PHYSICAL AND CHEMICAL PROPERTIES Substance Physical Property Chemical Property Helium Less dense than air Nonflammable Wood Grainy texture Flammable Baking soda White powder Reacts with vinegar to produce bubbles Rubbing alcohol Clear liquid Flammable Red food coloring Red color Reacts with bleach and loses color Iron Malleable Reacts with oxygen

CHEMICAL CHANGE A chemical change occurs when one or more substances are changed into entirely NEW substances with DIFFERENT properties

LAW OF CONSERVATION OF MASS Scientific Law that states mass can neither be created or destroyed in a physical or chemical reaction For example, when an antacid tablet reacts with water (alka seltzer) the mass of the water and the tablet before the reaction is equal to the mass of the remaining water and carbon dioxide gas after the reaction