Phase Changes Phase Changes We studied the three


























- Slides: 33

Phase Changes

Phase Changes We studied the three states of matter last week. They are? Solid Liquid Gas You know that matter can exist in each of these states but it’s dependent upon what? The energy of the molecules. As the energy increases, the molecules move around faster since they are less influenced by their bonds.

Phase Changes Today we are going to discuss how matter changes states. & how the kinetic theory applies to phase changes. As matter changes states it undergoes what’s called a phase change. Fundamentals behind this concept is used all the time and impacts your life in a variety of ways. Boiling water Trying to prevent the ice in your Polar Pop from melting.

Phase Changes Characteristics of Phase Changes When two states of the same substance are present each can be described as being in a different phase. Example: Ice shelf which is a solid and the run off water which is a liquid. A phase change is the reversible physical change that occurs when a substance changes from one state of matter to another.

Phase Changes Objectives: List and define six common phase changes. Describe what happens to a substance’s temperature and a systems energy during a phase change. Identify the arrangement of water molecules as a phase change occurs. Differentiate between evaporation and boiling.

Phase Changes Vocabulary: Phase change Endothermic Heat of fusion Exothermic Vaporization Heat of vaporization Evaporation Vapor pressure Condensation Sublimation Deposition

Phase Changes Six Common Phase Changes Melting Freezing Vaporization Condensation Sublimation Deposition

Phase Changes Melting The change from solid to a liquid. (Solid Liquid) Easy But, how do you propose this happens – using the kinetic theory? When you take an ice cube out of the freezer, energy in the form of heat flows from the air to the ice. As ice gains energy the molecules in the ice begins to vibrate faster and faster. At 0ºC the vibrations of water molecules become faster so that the attractions holding the molecules close together begin to break apart. When all molecules have become energized melting is complete and the ice has completed the transition to its liquid form.

Phase Changes Freezing is the opposite of melting, liquid to a solid. How does the kinetic theory apply? Place liquid in the freezer and energy leaves the water and enters the air. As kinetic energy decreases the molecules move more slowly. Soon the molecules become more influenced by their attractions to one another, vibrations slow and the molecules become fixed again. This completes freezing.

Phase Changes: Vaporization and Condensation Vaporization: it the change of a liquid to a gas. It an endothermic process. (What is meant by an endothermic process? ) Endothermic Process/Reaction: Vaporization absorbs energy from the surroundings to make this change. The amount of energy for a substance to change from a liquid to a gas is its Heat of Vaporization. There are two types of Vaporization Boiling: the entire volume is making the transition at the same time. This is different from evaporation Evaporation: takes the place at the surface of a liquid and occurs at temperatures below the boiling point.

Evaporation At room temperature some of the molecules of water have enough energy to completely break free of the hydrogen bonds that holds water molecules together in a fluid. When this happens, evaporation occurs.

Phase Changes In a closed container, liquids will still evaporate. When this happens in a closed container the released molecules collide with the container walls. The pressure cause by molecules colliding with the walls of the container is called: vapor pressure.

Vapor Pressure Evaporation creates vapor pressure as the evaporating water molecules run into the sides of the closed container. Try this. Get a jar with a tight fitting lid. Fill with an inch or so of water, fasten the lid, and leave in the sun for a few hours. Slowly open the lid and listen for the ‘ssss’ This is the vapor Vapor Pressure Due to the collisions between the water molecules & the sides of the container.

Phase Changes: Boiling When heating a liquid, such as water, the temp and the vapor pressure both increase. When vapor pressure (an upward force) becomes equal to or greater than atmospheric pressure (gravity pulling down on molecule) water boils. As temperature increases, water molecules move faster and faster, increasing the kinetic energy. When the temperature reaches 100ºC molecules below the surface have enough kinetic energy to overcome the attraction of other molecules. This causes vapor formed at the bottom to rise to the top creating bubbles and to be released

Phase Changes: Factors affecting boiling point. Boiling Continued: The boiling point of a substance depends on the atmospheric pressure. At sea level water boils at 100ºC But at higher elevations atmospheric pressure decreases Therefore, in places like Denver (The Mile High City) water can boil at a lower temp (95ºC). It is boiling at a lower temp but that doesn’t mean it’s hotter. If fact, it takes longer to cook at higher elevations because the boiling point is lower.


Phase Changes Condensation: the phase change in which a substance changes from a gas or vapor to a liquid This is the opposite of vaporization. This is seen on the sides of cold glasses during monsoon season, on the doors of showers, and the inside of steamy windows. How does the kinetic theory apply? . When vapor comes into contact with the sides of a cold glass, it loses kinetic energy and the attractions between water

Add More energy (Heat to boiling) H 2 O as Water Vapor: A lot of Kinetic Energy Condensing Freezing H 2 O as water: Medium Kinetic Energy Boiling Add energy (Heat to melting) Melting Water As Ice: Low Kinetic Energy

Phase Changes The Two Extreme Cases Sometimes phase changes can happen but they skip the intermediate steps Sublimation: the phase change in which a substance changes from a solid to a gas or vapor. An extremely endothermic process. Dry ice sublimes. It releases CO 2 into the environment as it takes in a large quantity of kinetic energy very quickly. Observe. Deposition: the phase change when a gas or vapor changes directly to a solid without changing to a liquid. An extremely exothermic process, gas particles can lose almost all of their kinetic energy and form a solid almost instantly. Frost forming on ice cold windows is an example of deposition.

Phase Changes: Concept Check What are their opposites? Melting Sublimation Vaporization Deposition Freezing Condensation What are their opposites? Freezing Deposition Condensation Sublimation Melting Vaporization

Phase Changes: Temperature and Phase Changes. Phase change can be determined by checking the temperature as the substance is heated or cooled. There is something interesting and obvious about a phase change. The temperature of a substance does not change during a phase change. ? ? ? This may be counterintuitive but it’s true. As ice melts it doesn’t change temperature until all the ice has melted. We will explore this but take my word on this until then.

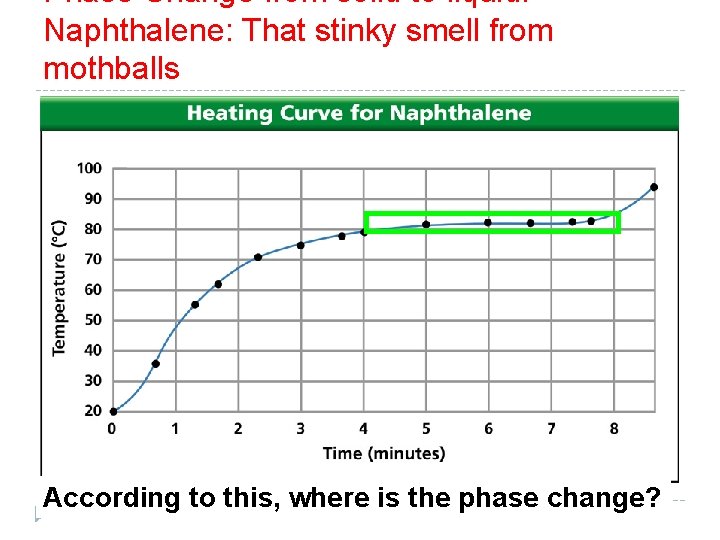
Phase Change from solid to liquid: Naphthalene: That stinky smell from mothballs According to this, where is the phase change?

Remember some facts… When a solid is heated and completely turns into a liquid then it has melted. The temperature at which this occurs is the melting point. The temperature at which a liquid completely turns into a solid is its freezing point. The temperature at which a substance boils is the boiling point and at the boiling point the substance may become a vapor. Question: Who can tell me what type of property these facts represent? Correct! Physical Properties.

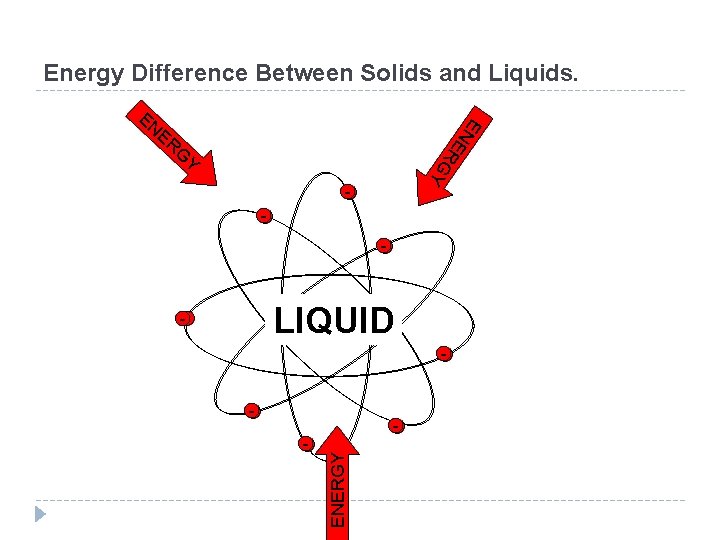
Phase Changes Energy and Phase Change During a phase change ENERGY is transferred. From substance to surroundings Or for the surroundings (like a heat source) to substance. Direction is dependent upon the type of phase change. Regardless: Energy is either absorbed or released during a phase change.

Energy Difference Between Solids and Liquids. ER EN E EN G R G Y Y +++ LIQUID SOLID -- ENERGY - -

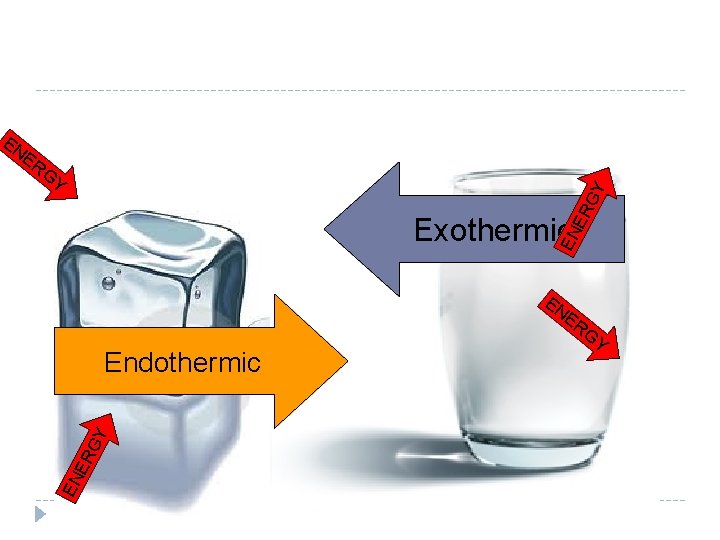
Descriptions of Types of Phase Changes Phase changes can occur in two directions. One way the matter is gaining energy from the surroundings. This is called an endothermic change. In an endothermic change the system (the material) ABSORBS energy from its surroundings Think of “endo” as “into” Heating ice to melt it into water is an example of this.

Descriptions of Types of Phase Changes Phase changes going the other direction involves the matter giving energy to the surroundings or the surroundings taking energy from the matter. Water freezing is an example of this. RELEASE of energy to its surroundings during a phase change is called an Exothermic Change. Think of “exo” as “exits” During freezing, the freezer (or cold environment) takes energy from the matter.

ER GY GY EN Exothermic EN ER ER GY Endothermic EN EN ER GY

Phase Changes Energy and Phase Changes continued: Energy absorbed during melting equals energy released when freezing. The amount of energy needed for phase changes is dependent upon the substance (it’s a physical property). Some absorb and release more than others. This is called water’s Heat of Fusion: The energy required to melt the substance. Fusion is another way of saying melting. As water freezes it releases 334 joules (J) of energy. 1 gram of ice absorbs 334 joules (J) of energy when it melts. So. The energy needed for ice (to melt to) water equals

THE EXOTHERMIC REACTION AND ENDOTHERMIC REACTION HAVE EQUAL BUT OPPOSITE ENERGY TRANSFER ER GY GY EN Exothermic EN ER ER GY Endothermic EN EN ER GY

Summary: Exo- and Endothermic Reactions a. liquid b. liquid c. gas d. liquid e. gas f. gas

Phase Changes Summary 6 common phase changes: melting, freezing, condensation, vaporization, sublimation, and deposition Temperature does not change through a phase change Energy is either absorbed or released during a phase change Boiling is a phase change in which water molecules undergo vaporization and is released, whereas evaporation can result in the loss of energetic water molecules below the boiling point of water.

Complete the Diagram