Properties and Classification of Matter Properties of Matter






















- Slides: 37

Properties and Classification of Matter

Properties of Matter Physical Properties Chemical Properties • Any characteristic that you can observe without changing the makeup of the material. • Any characteristic that gives the substance the ability to change into another substance. • Ex. Mass, weight, color, volume, density, odor, shape, texture, hardness, boiling pt. , melting pt. • Ex. Reactive to light? , flammability, p. H(acid/base), electronegativity, ability to oxidize • We’ll discuss

Mass • Amount of matter in an object. • Measure with a triple beam balance. – Unit is Kilogram(kg)

Weight • Measure of the pull of gravity on an objects mass. • Measure with a scale. – Unit is the Newton(N)

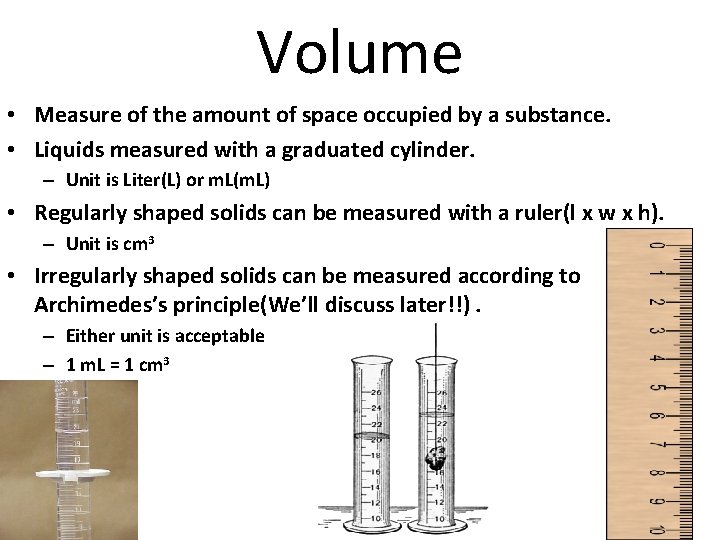
Volume • Measure of the amount of space occupied by a substance. • Liquids measured with a graduated cylinder. – Unit is Liter(L) or m. L(m. L) • Regularly shaped solids can be measured with a ruler(l x w x h). – Unit is cm 3 • Irregularly shaped solids can be measured according to Archimedes’s principle(We’ll discuss later!!). – Either unit is acceptable – 1 m. L = 1 cm 3

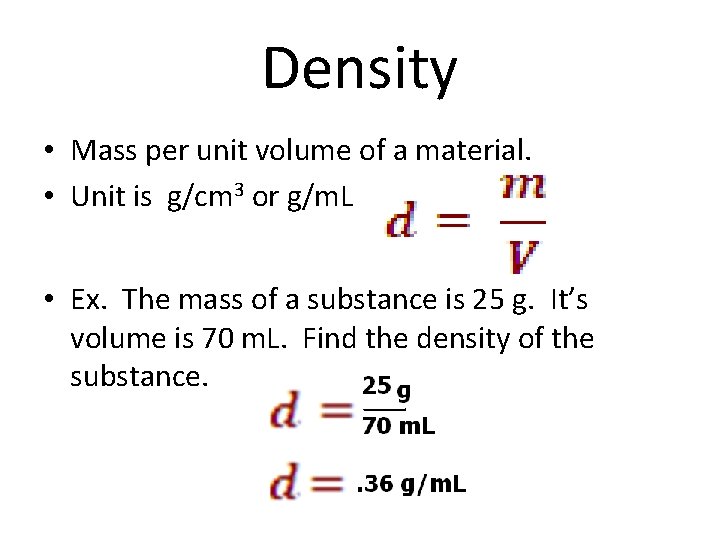
Density • Mass per unit volume of a material. • Unit is g/cm 3 or g/m. L • Ex. The mass of a substance is 25 g. It’s volume is 70 m. L. Find the density of the substance.

2 Types of Physical Properties Extensive Properties • Those that do depend on the size of the sample involved. • Some of the most common types of extensive properties are; length, volume, mass and weight. Intensive Properties • Those that do not depend on the size of the sample involved. • Some of the most common intensive properties are; density, freezing point, melting point and color.

Physical Properties used to identify a Substance • In order to use a physical property to identify a substance, that property must be unique to that substance. » “Entire list would be exhaustive” » Simply ask yourself “Is it unique to that substance!!”

Can you use color to identify a substance?

NO

Can you use melting point to identify a substance?

It is unique for every substance!!!

Can you use Volume to identify a substance?

NO

Can you use mass to identify a substance?

NO

Can you use density to identify a substance?

It is unique for every substance!!!

Can you use Shape to identify a substance?

NO

Can you use boiling point to identify a substance?

It is unique for every substance!!!

Which of the following physical properties could be used to identify a substance? A. B. C. D. Color Weight Mass Melting Point

Which of the following is not considered a physical property? A. B. C. D. Mass Color p. H Volume

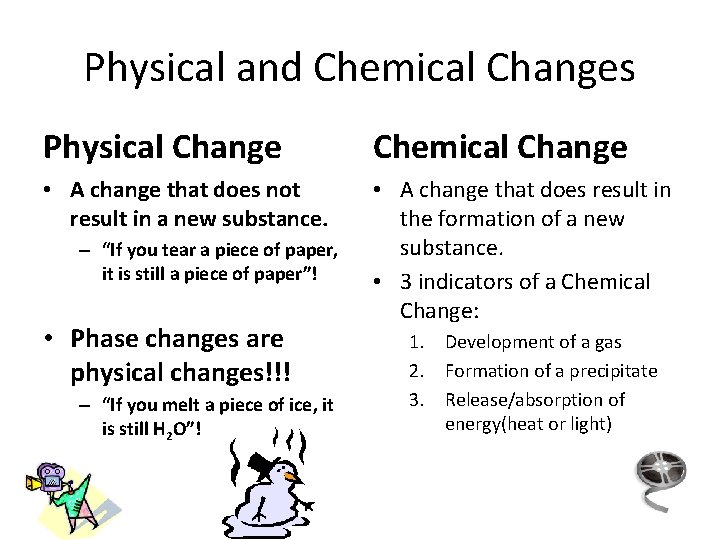
Physical and Chemical Changes Physical Change Chemical Change • A change that does not result in a new substance. • A change that does result in the formation of a new substance. • 3 indicators of a Chemical Change: – “If you tear a piece of paper, it is still a piece of paper”! • Phase changes are physical changes!!! – “If you melt a piece of ice, it is still H 2 O”! 1. Development of a gas 2. Formation of a precipitate 3. Release/absorption of energy(heat or light)

Indicators of Chemical Change(cont): 1. Development of a gas – Must be a “new” gas – Cannot be a gas that was already present!! – If you shake a carbonated drink, the “fizz” you hear when you open it is a gas that was already dissolved in the drink. Not a new gas!!!

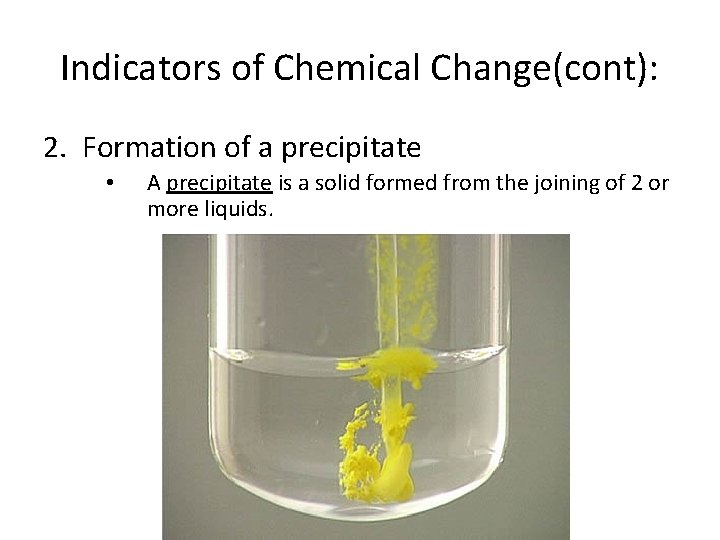
Indicators of Chemical Change(cont): 2. Formation of a precipitate • A precipitate is a solid formed from the joining of 2 or more liquids.

Indicators of Chemical Change(cont): 3. Release/absorption of energy(heat or light) – Endothermic vs. Exothermic Reactions • Endothermic Reactions—take in heat • Exothermic Reactions—give off heat

Indicators of Chemical Change(cont. ) • Sometimes changes in color are associated with chemical changes. – Must be careful!! Sometimes they are physical changes. – Ask yourself, “Is there a new substance produced? ? ”

Which of the following is Not an indicator of a chemical change? A. A precipitate is formed B. A new gas is released C. The object changes shape D. Object gives off light
In which of the following did a chemical change occur? A. B. C. D. A watermelon is cut in half! Your snowman melts!!! A marshmallow is burnt in a fire!! A bicycle tire goes flat!!

In which of the following did a chemical change occur? A. B. C. D. A piece of paper is torn in half! An iron nail rusts! A pencil is sharpened! A puddle of water evaporates into the sky!!

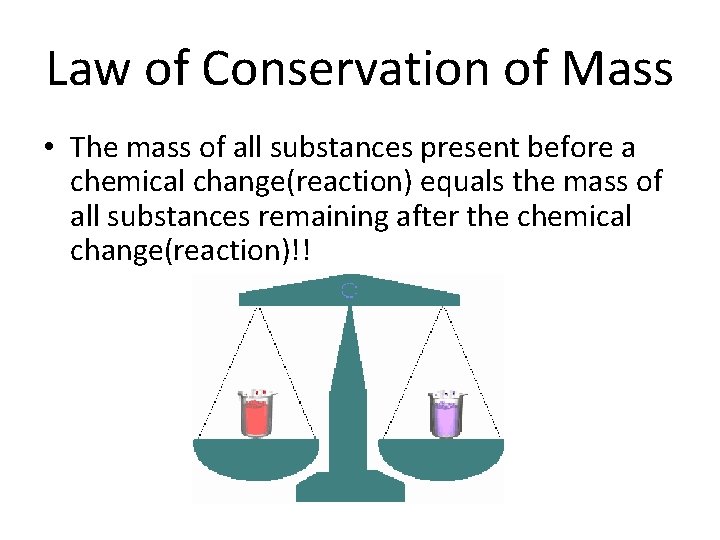
Law of Conservation of Mass • The mass of all substances present before a chemical change(reaction) equals the mass of all substances remaining after the chemical change(reaction)!!

Classification of Matter Chuck, you don’t know what matter is, How in the world would you know how it’s classified?

Classifying matter is just like classifying football plays. There are run plays , pass plays, plays where you fake the run and then pass………

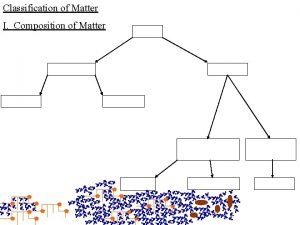
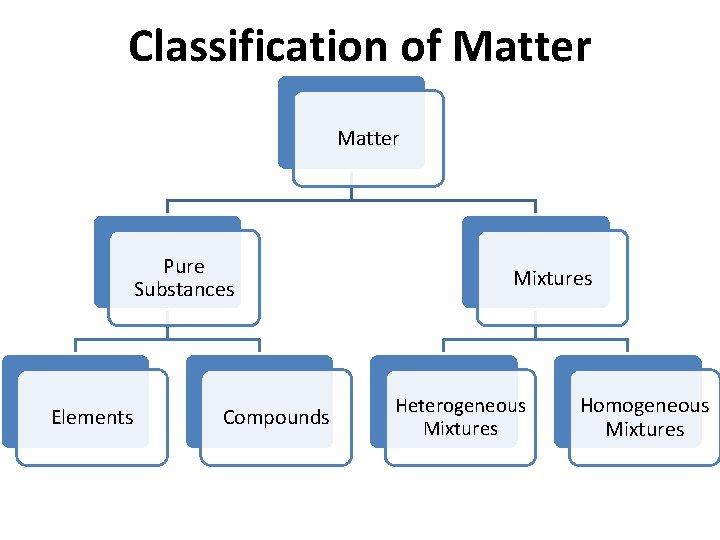
Classification of Matter Pure Substances Elements Compounds Mixtures Heterogeneous Mixtures Homogeneous Mixtures

In Notebook, answer questions 1 -5 p. 465
 Classification and properties of matter
Classification and properties of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Section 1 composition of matter
Section 1 composition of matter Grey matter
Grey matter Brain falx
Brain falx Gray matter and white matter
Gray matter and white matter Ncl caudatus
Ncl caudatus Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter and its properties
Matter and its properties The study of composition structure and properties of matter
The study of composition structure and properties of matter Matter-properties and changes answer key
Matter-properties and changes answer key Properties and characteristics of matter
Properties and characteristics of matter Which is a big idea for matter and change
Which is a big idea for matter and change Material useful and harmful
Material useful and harmful Natural science grade 7 lesson plans term 2
Natural science grade 7 lesson plans term 2 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Extensive and intensive properties
Extensive and intensive properties Physical and chemical properties
Physical and chemical properties Classification of tabulation
Classification of tabulation 2 properties of liquids
2 properties of liquids Properties of matter vocabulary
Properties of matter vocabulary Composition of matter concept map
Composition of matter concept map Objectives of properties of matter
Objectives of properties of matter General properties of matter
General properties of matter Measurable properties of matter
Measurable properties of matter Properties of matter jeopardy
Properties of matter jeopardy Matter jeopardy
Matter jeopardy Matter definition
Matter definition Graphic organizer classification
Graphic organizer classification What are some chemical properties of matter
What are some chemical properties of matter Properties of matter section 2
Properties of matter section 2 Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Properties of matter vocabulary
Properties of matter vocabulary Properties of matter grade 7
Properties of matter grade 7 General properties of matter
General properties of matter Which is a “big idea” of physical science?
Which is a “big idea” of physical science?