PROPERTIES OF MATTER Identify the characteristics of matter














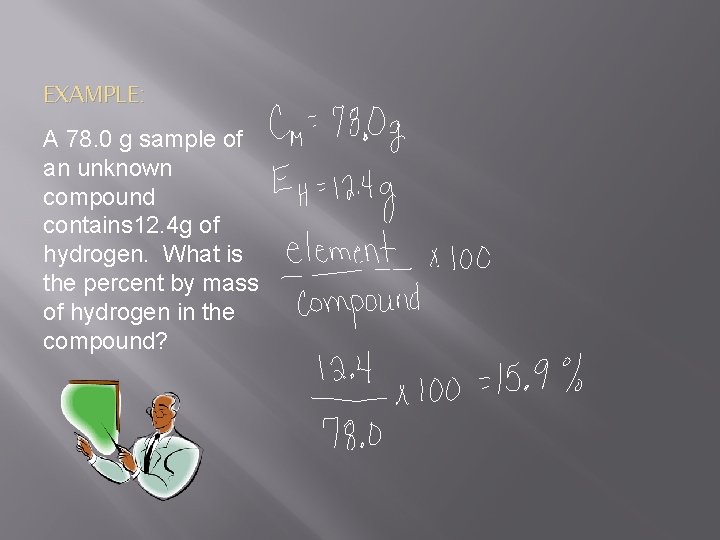






















- Slides: 65

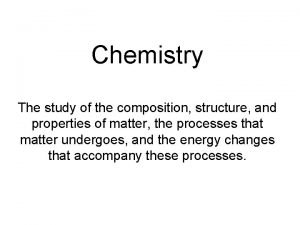
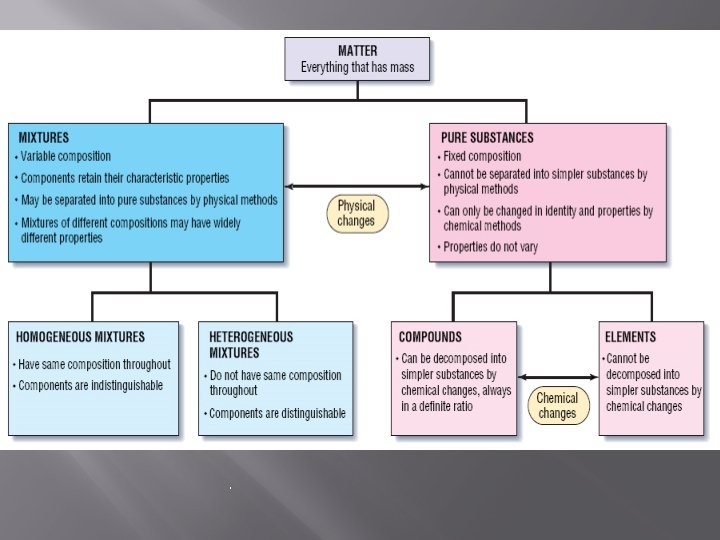
PROPERTIES OF MATTER � � Identify the characteristics of matter and substances Differentiate among the three states of matter Define physical properties of substances � Matter is anything that has mass and takes up space. Mass of an object is the amount of matter the object contains. Matter that has a uniform and definite composition is called a substance � Pure substances contain only one kind of matter Non example: lemonade �Discussion with shoulder buddy on why lemonade is not an example.


Diagrams of Matter

substance ELEMENTS � � The simplest forms of matter that can exist under normal laboratory conditions Represented by a unique chemical name and chemical symbols Organized on the periodic table Examples: oxygen, hydrogen, and carbon COMPOUNDS � � Substances that can be separated into simpler substances only by chemical means. Made up of 2 or more different elements that are combined chemically in a set ratio Separating a compound requires energy such as heat or electricity. Example: salt, baking soda, sugar

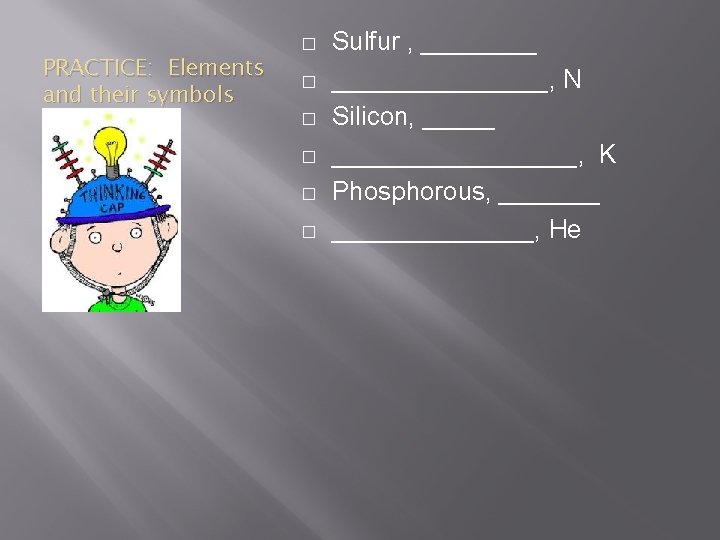
PRACTICE: Elements and their symbols � � � Sulfur , _______________, N Silicon, ___________, K Phosphorous, ______________, He

MIXTURE Mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties � Most everyday matter occurs as a mixture � Composition of mixtures is variable � Substances tend to mix naturally (hard to keep pure) � Can be separated by physical means

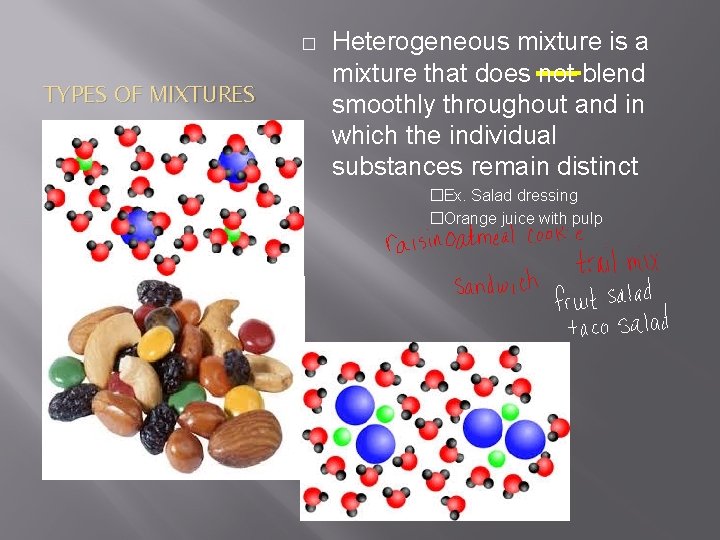
� TYPES OF MIXTURES Heterogeneous mixture is a mixture that does not blend smoothly throughout and in which the individual substances remain distinct �Ex. Salad dressing �Orange juice with pulp

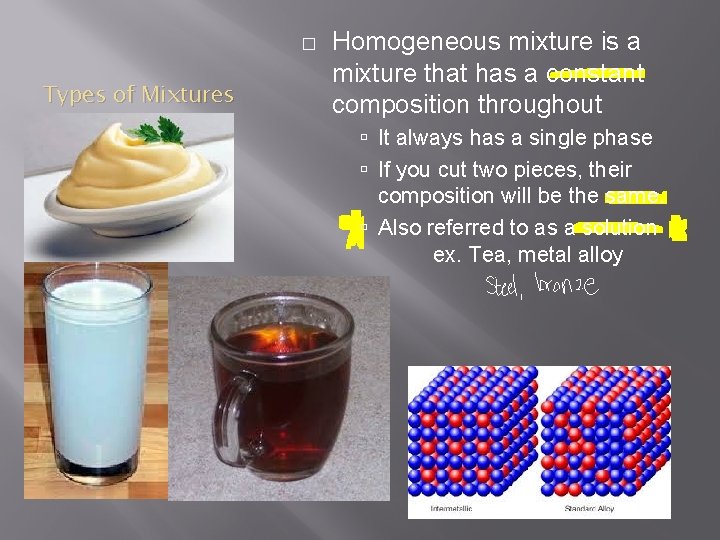
� Types of Mixtures Homogeneous mixture is a mixture that has a constant composition throughout It always has a single phase If you cut two pieces, their composition will be the same. Also referred to as a solution ex. Tea, metal alloy

Compare mixtures and substances � Create a Venn Diagram

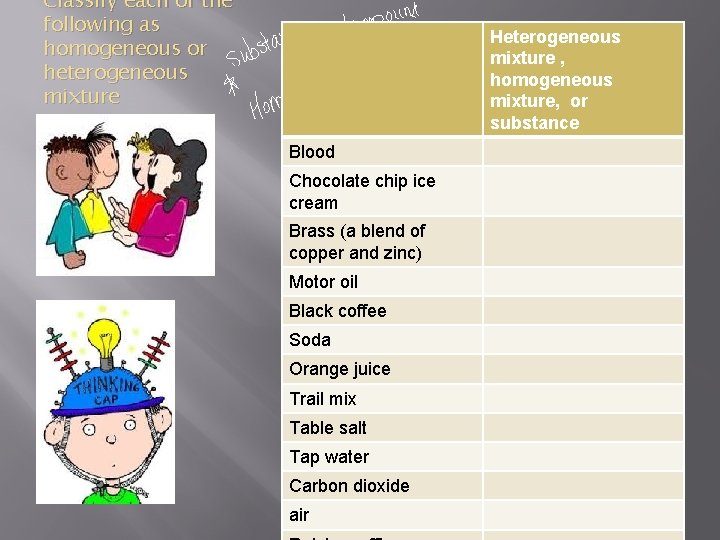
Classify each of the following as homogeneous or heterogeneous mixture Heterogeneous mixture , homogeneous mixture, or substance Blood Chocolate chip ice cream Brass (a blend of copper and zinc) Motor oil Black coffee Soda Orange juice Trail mix Table salt Tap water Carbon dioxide air

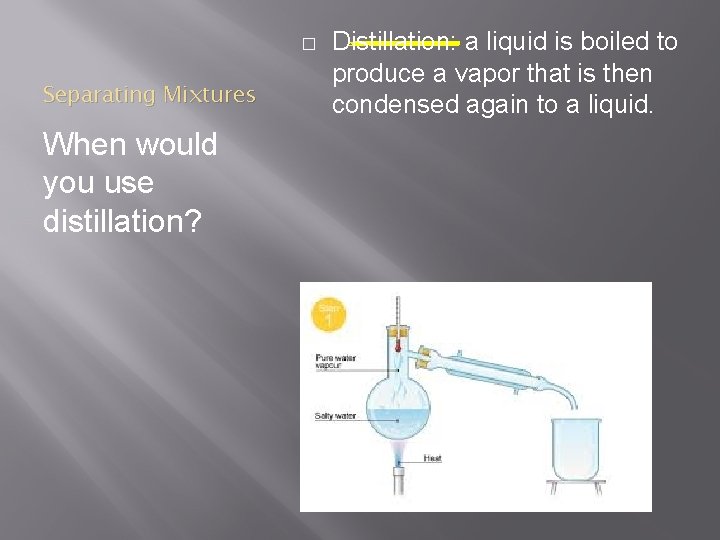
� Separating Mixtures When would you use distillation? Distillation: a liquid is boiled to produce a vapor that is then condensed again to a liquid.

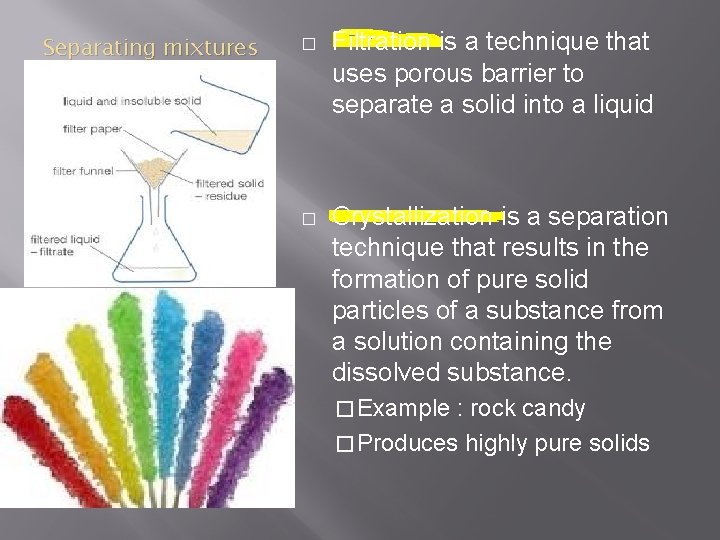
Separating mixtures � Filtration is a technique that uses porous barrier to separate a solid into a liquid � Crystallization is a separation technique that results in the formation of pure solid particles of a substance from a solution containing the dissolved substance. � Example : rock candy � Produces highly pure solids

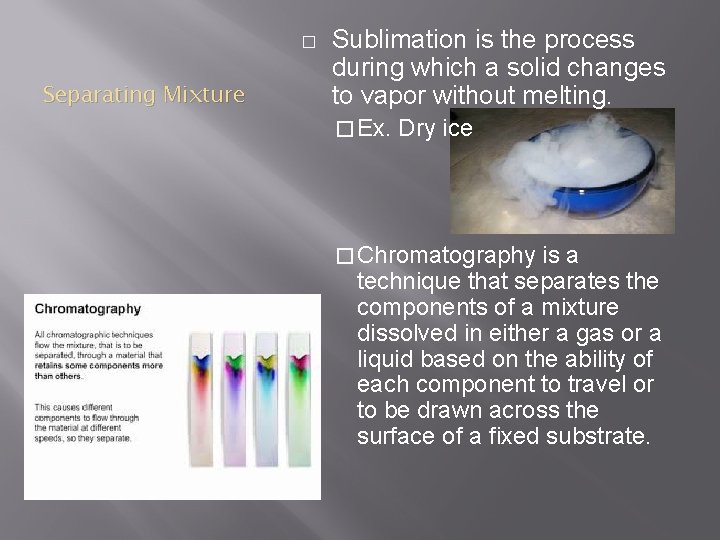
� Separating Mixture Sublimation is the process during which a solid changes to vapor without melting. � Ex. Dry ice � Chromatography is a technique that separates the components of a mixture dissolved in either a gas or a liquid based on the ability of each component to travel or to be drawn across the surface of a fixed substrate.

� THINK and REFLECT Describe the separation technique that could be used to separate each of the following mixtures � Two colorless liquids � A non-dissolving solid mixed with a liquid � Red and blue marbles of the same size and mass � Sulfur and iron � Sand salt � Gasoline and water � Aluminum and steel � Copper and silver

PROPERTIES OF MATTER � Physical property is a quality or condition of a substance that can be observed or measured without changing the substance’s composition � Examples: Color, solubility, odor, hardness, density, melting point, boiling point

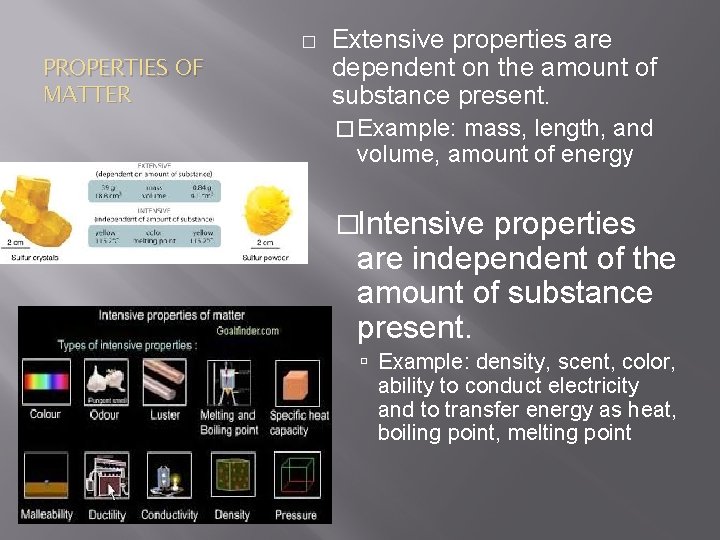
� PROPERTIES OF MATTER Extensive properties are dependent on the amount of substance present. � Example: mass, length, and volume, amount of energy �Intensive properties are independent of the amount of substance present. Example: density, scent, color, ability to conduct electricity and to transfer energy as heat, boiling point, melting point

Classify if the property is intensive or extensive property Intensive or extensive property Color Smells like vanilla Length Boiling point Ability to attract a magnet Density mass

PROPERTIES OF MATTER � Chemical Property is the ability or inability of a substance to combine with or change into one or more other substances. � Example: iron forming rust when combined with oxygen.

Classify the following as physical or chemical properties Physical or Chemical Property Iron and oxygen form rust Iron is more dense than aluminum Magnesium burns brightly when ignited Oil and water do not mix Mercury melts at -39 C Water has a density of 1. 00 g/cm 3 Blue gray color brittle Reacts vigorously with fluorine

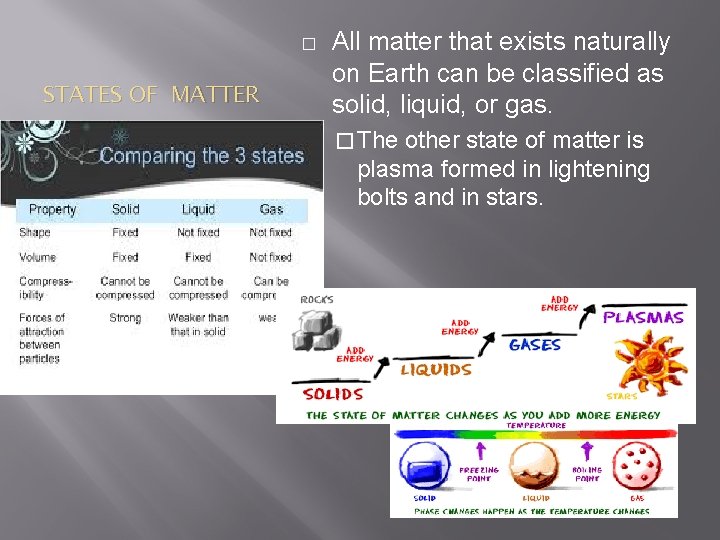
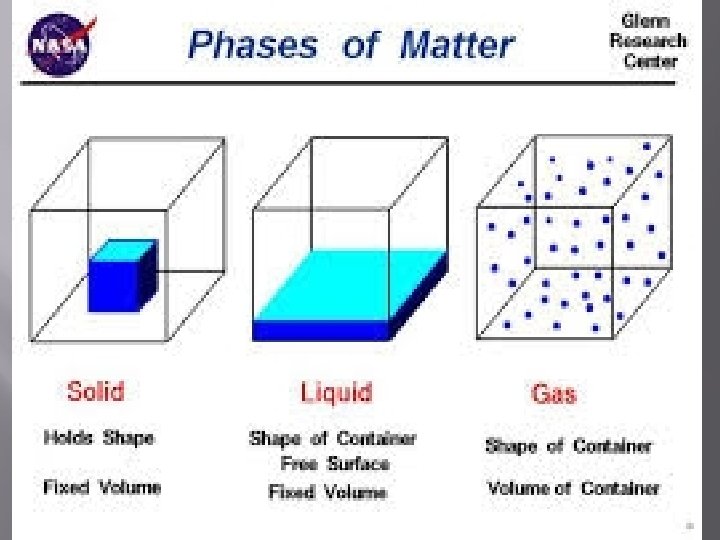
� STATES OF MATTER All matter that exists naturally on Earth can be classified as solid, liquid, or gas. � The other state of matter is plasma formed in lightening bolts and in stars.


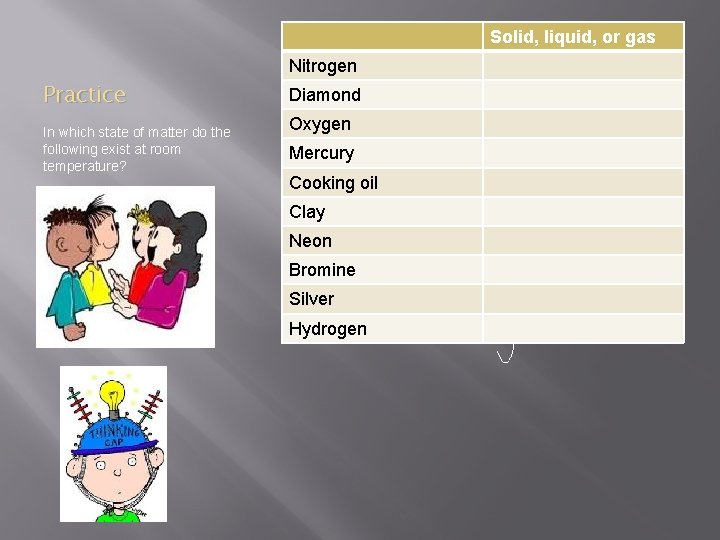
Solid, liquid, or gas Nitrogen Practice In which state of matter do the following exist at room temperature? Diamond Oxygen Mercury Cooking oil Clay Neon Bromine Silver Hydrogen

� Fingernail polish remover (mostly acetone) is a liquid at room temperature. Would you describe acetone in the gaseous state as a vapor or a gas? � Justify your answer THINK

� THINK PAIR SHARE ILLUSTRATION AND DIAGRAMMING TIME!!! Compare the properties of solids, liquids, and gases. Make a table that describes shape, volume, compressibility, and structure to share with the class.

Physical Change What is a physical change? A change which alters a substance without changing its composition is a physical change. Example: cutting paper, chopping wood, and freezing water.

Chemical Change What is a chemical change? � A process that involves one or more substances changing into new substances New substances formed in the reaction have a different composition and different properties from the substances before the reaction � Ability of a substance to undergo a chemical reaction �

Evidence of a Chemical Reaction How do we know a chemical change has taken place? � A chemical reaction always produces a change in properties. � Change in temperature � Gas produced � Precipitate formed � Change in color � Change in odor

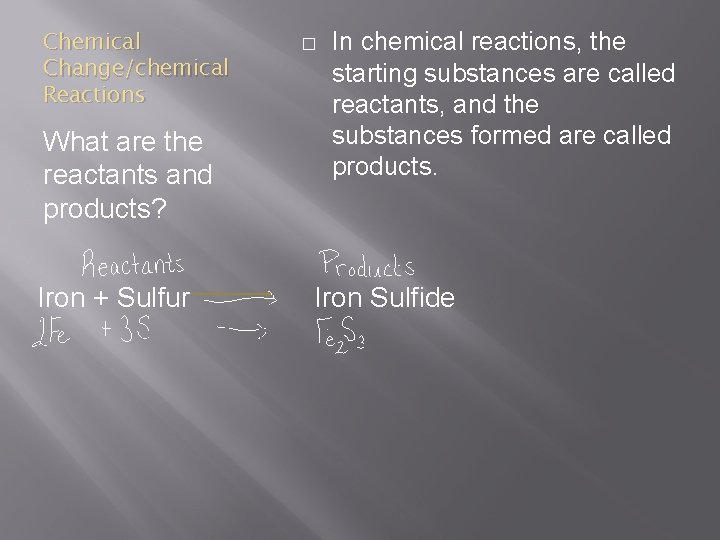
Chemical Change/chemical Reactions What are the reactants and products? Iron + Sulfur � In chemical reactions, the starting substances are called reactants, and the substances formed are called products. Iron Sulfide

� CREATION TIME CREATE A T-CHART of Chemical and Physical changes

� THINK PAIR SHARE State the difference between a physical change and a chemical change, and list three likely indications that a chemical reaction has occurred. Which indication is most suggestive of a chemical reaction?

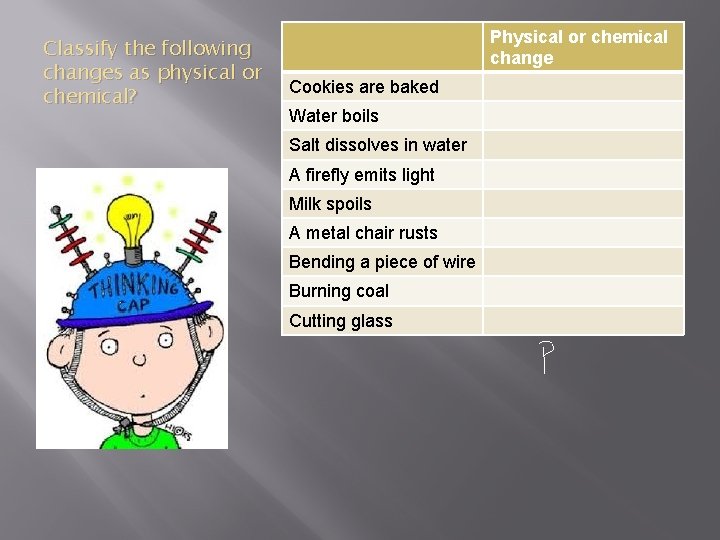
Classify the following changes as physical or chemical? Physical or chemical change Cookies are baked Water boils Salt dissolves in water A firefly emits light Milk spoils A metal chair rusts Bending a piece of wire Burning coal Cutting glass

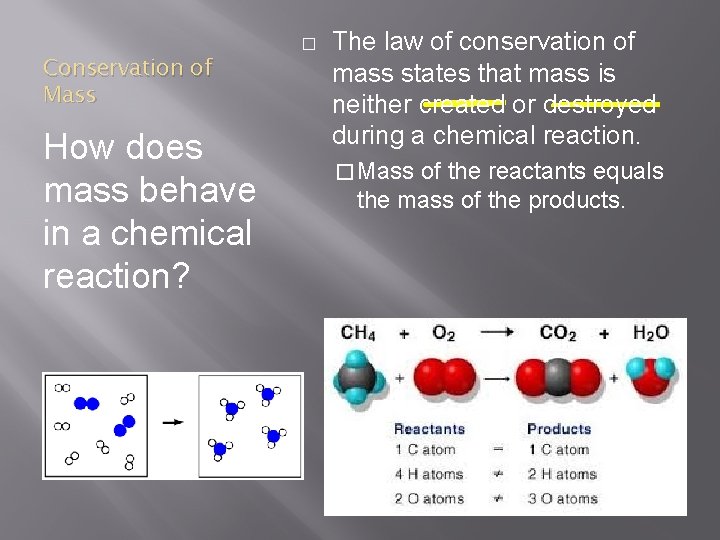
Conservation of Mass How does mass behave in a chemical reaction? � The law of conservation of mass states that mass is neither created or destroyed during a chemical reaction. � Mass of the reactants equals the mass of the products.

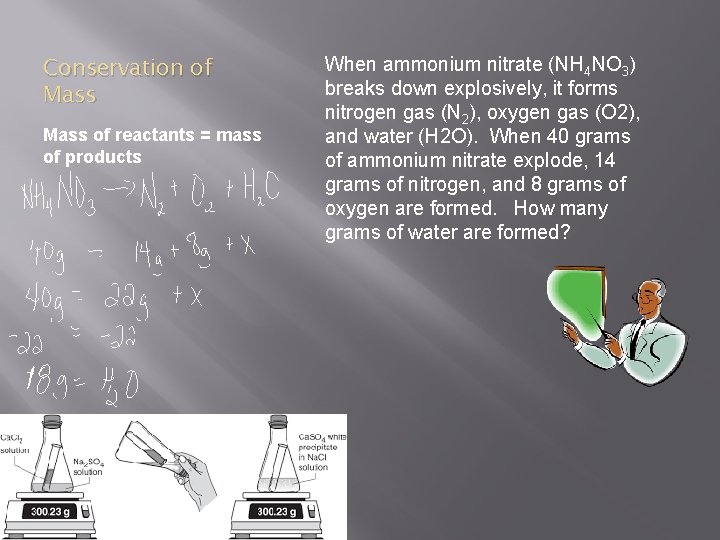
Conservation of Mass of reactants = mass of products When ammonium nitrate (NH 4 NO 3) breaks down explosively, it forms nitrogen gas (N 2), oxygen gas (O 2), and water (H 2 O). When 40 grams of ammonium nitrate explode, 14 grams of nitrogen, and 8 grams of oxygen are formed. How many grams of water are formed?

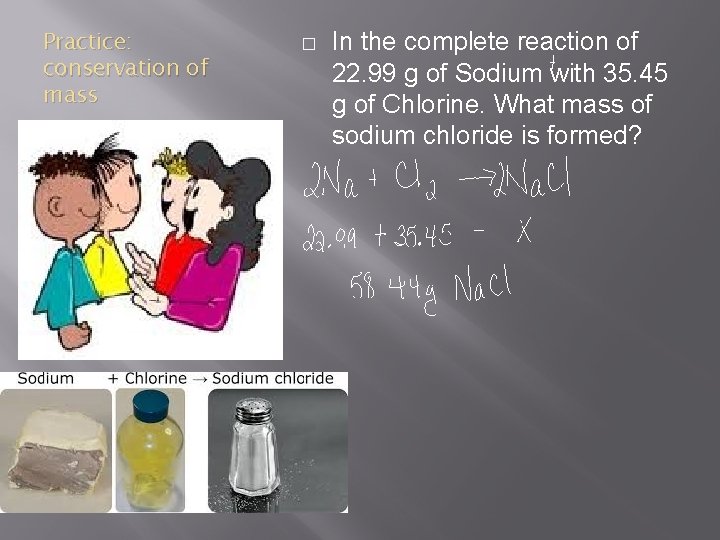
Practice: conservation of mass � In the complete reaction of 22. 99 g of Sodium with 35. 45 g of Chlorine. What mass of sodium chloride is formed?

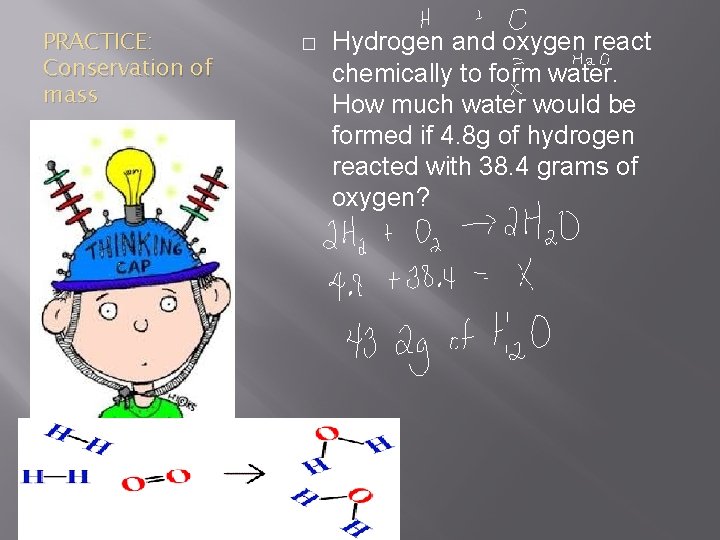
PRACTICE: Conservation of mass � Hydrogen and oxygen react chemically to form water. How much water would be formed if 4. 8 g of hydrogen reacted with 38. 4 grams of oxygen?

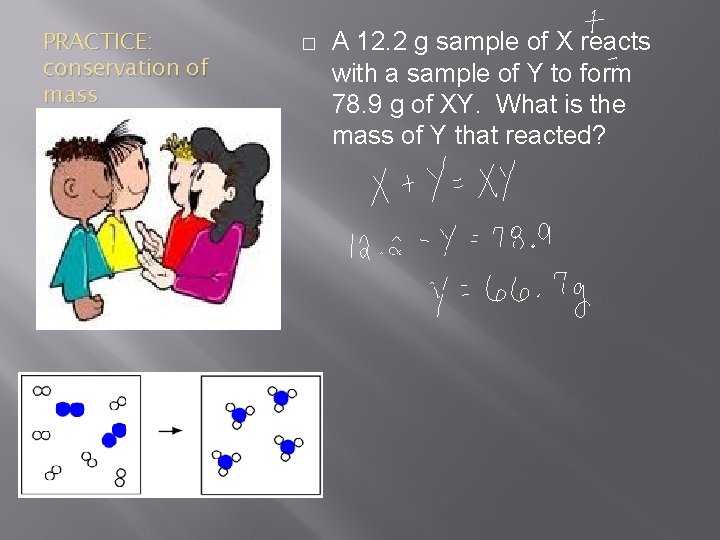
PRACTICE: conservation of mass � A 12. 2 g sample of X reacts with a sample of Y to form 78. 9 g of XY. What is the mass of Y that reacted?

THINK: Conservation of mass When powered iron is left exposed to air, it rusts. Explain why the rust weighs more than the original powered iron?

Law of Definite Proportions � The law of definite proportions states that a compound is always composed of the same elements in the same proportion by mass, no matter how large or small the sample. � The mass of the compound is equal to the sum of the masses that make up the compound.

� The percent by mass is the ratio of the mass of each element to the total mass of the compound expressed as a percentage � Percent by mass = Percent by mass �Percent by mass is obtained by dividing the mass of the element by the mass of the compound and then by multiplying this ratio by 100 to express it as a percentage.
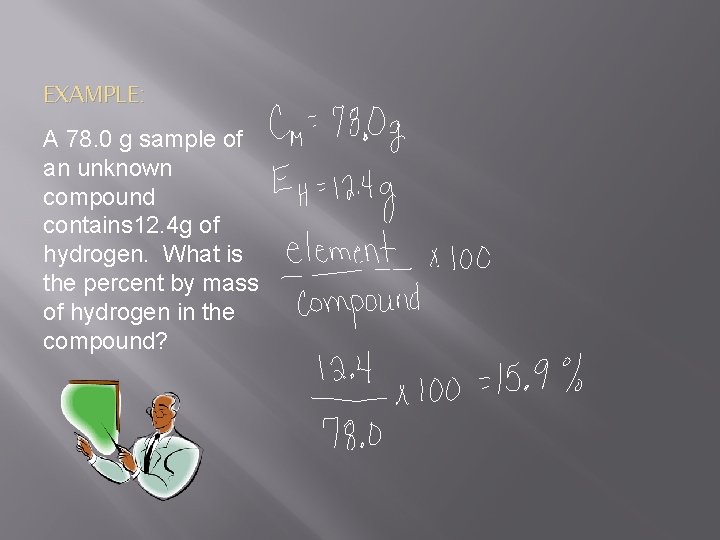
EXAMPLE: A 78. 0 g sample of an unknown compound contains 12. 4 g of hydrogen. What is the percent by mass of hydrogen in the compound?

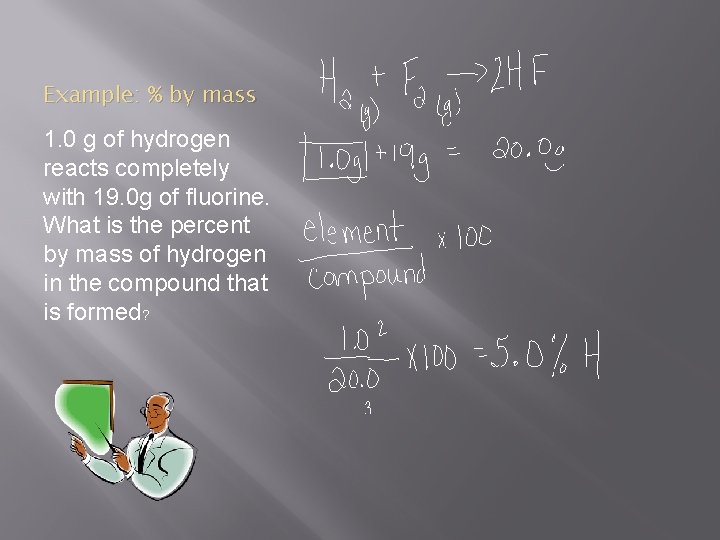
Example: % by mass 1. 0 g of hydrogen reacts completely with 19. 0 g of fluorine. What is the percent by mass of hydrogen in the compound that is formed?

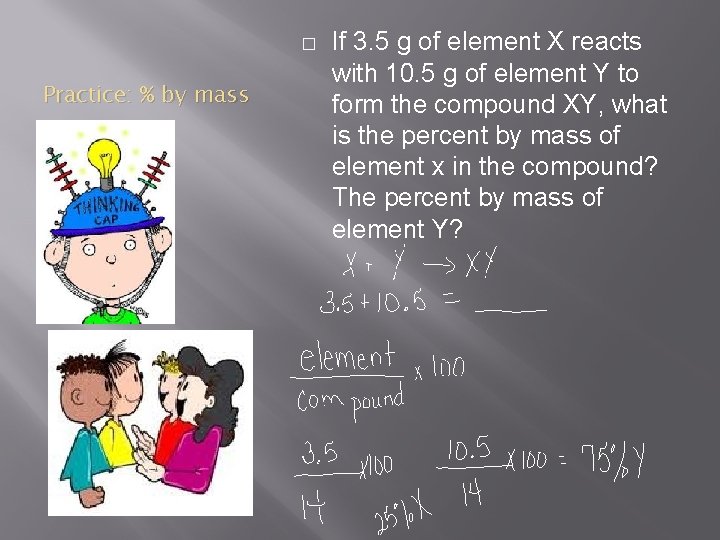
� Practice: % by mass If 3. 5 g of element X reacts with 10. 5 g of element Y to form the compound XY, what is the percent by mass of element x in the compound? The percent by mass of element Y?

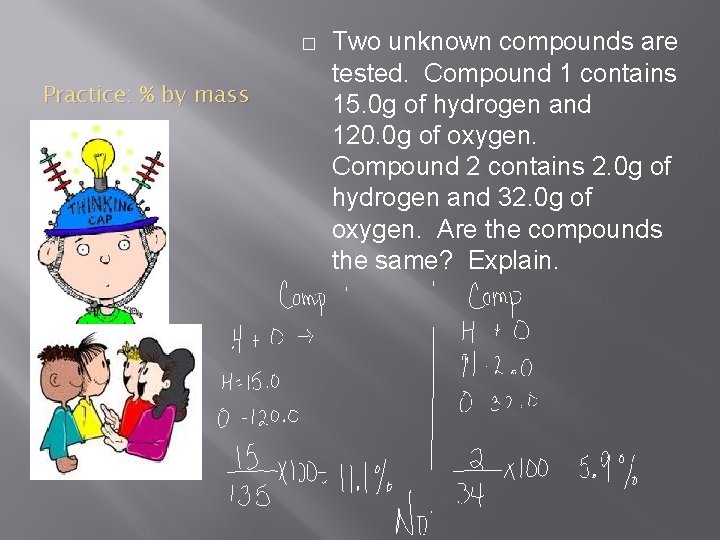
� Practice: % by mass Two unknown compounds are tested. Compound 1 contains 15. 0 g of hydrogen and 120. 0 g of oxygen. Compound 2 contains 2. 0 g of hydrogen and 32. 0 g of oxygen. Are the compounds the same? Explain.

Law of multiple Proportions � The law of multiple proportion states that when different compounds are formed by a combination of the same elements, different masses of one element combine with same fixed mass of the other element in a ratio of small whole numbers. Example: water and hydrogen peroxide �H 2 O = 2: 1 �H 2 O 2 = 2: 2

REVIEW QUESTIONS � Describe a method that could be used to separate each mixture � Iron fillings and sand � Sand � The and salt components of ink � Helium and oxygen gases

Review Questions � Which of the following are the same and which are different? Justify your decision. �A substance and a pure substance � A heterogeneous mixture and a solution � A substance and a mixture � A homogeneous mixture and a solution

Correct the following statements � An element is a combination of two or more compounds � When a small amount of sugar is completely dissolved in water, a heterogeneous solution is formed.

Review Questions � Name the elements contained in the following compounds. � Sodium Chloride (Na. Cl) � Ammonia (NH 3 � Ethanol (C 2 H 6 O) � Bromine (Br 2

Review Questions � What is the percent by mass of carbon in 44 g of carbon dioxide?

Review Questions � What is the percent by mass of oxygen in 44 g of carbon dioxide?

Review Questions � A 25. 3 g sample of an unknown compound contains 0. 8 g of oxygen. What is the percent by mass of oxygen in the compound?

Review Questions � Magnesium combines with oxygen to form magnesium oxide. If 10. 57 g of magnesium reacts completely with 6. 96 g of oxygen, what is the percent by mass of oxygen in magnesium oxide?

Review Questions � Express the following numbers in scientific notation. � 34, 500 � 2665 � 0. 9640 � 789 � 75, 600 � 0. 002189

Review Questions � Perform the following mathematic problems using the rules for significant figures. � 2. 015 + 3. 1 +332. 41 = 33 * 1. 3021 = 1. 34 /0. 01 = 3444. 12 -3. 222 -122. 2 = � � �

Review Question � � Analyze Is gas escaping from an opened soft drink an example of a chemical or physical change? Explain

Review Questions � List physical properties of eggs before and after they are cooked. Based on your observations, does a physical change or chemical change occur when eggs are cooked? Justify your answer.

Review Questions � You might have noticed that while eating ice cream on a hot day, some of the ice cream begins to melt. Is the observed change in the state of ice cream a physical or chemical change? Justify your answer.

Review Question � Which states of matter are compressible? � Which states of matter are not compressible? � Explain.

Review Question � Phosphorous combines with hydrogen to form phosphine. In this reaction, 108. 3 g of phosphorous combines with excess hydrogen to produce 129. 9 g of phosphine. After the reaction, 11. 0 g of hydrogen remains unreacted. What was the initial mass of hydrogen before reaction? What mass of hydrogen is used in the reaction?

Review Questions � If you have 100 particles of hydrogen and 100 particles of oxygen, how many units of water can you form? Will you use all of the particles of both elements? If not, what will remain? �Hint: Chemical formula H 2 O

Review Questions � Which of the following is not a chemical change? � Paper being shredded � Steel rusting � Charcoal burning � A newspaper yellowing in the sun

Review Questions � Which of these properties could not be used to distinguish between table salt and table salt? � Boiling point � Melting point � Density � color

Review Questions � The state of matter characterized by a definite volume and an indefinite shape is � Solid � Mixture � Liquid � Gas

Review Question � Initial: final Product 2. Mixture 3. Matter 4. compound 1. Reactant: _____

Review Questions � Words: sentence 1. 2. 3. 4. Reactant Theory Compound Substance Elements : _____
 Properties and characteristics of matter
Properties and characteristics of matter Properties used to identify substances
Properties used to identify substances Identify which is matter
Identify which is matter Extensive vs intensive quantity
Extensive vs intensive quantity Chemical and physical properties
Chemical and physical properties Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thang điểm glasgow
Thang điểm glasgow Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Properties of liquid matter
Properties of liquid matter Properties of matter vocabulary
Properties of matter vocabulary Concept map properties of matter
Concept map properties of matter Objectives of properties of matter
Objectives of properties of matter Common properties of matter
Common properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Examples of chemical properties of matter
Examples of chemical properties of matter States of matter jeopardy
States of matter jeopardy Properties of matter jeopardy
Properties of matter jeopardy Properties of matter definition
Properties of matter definition Matter and its properties
Matter and its properties Classifying matter graphic organizer
Classifying matter graphic organizer The study of composition structure and properties
The study of composition structure and properties Chemical properties of matter
Chemical properties of matter 2 properties of matter
2 properties of matter Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Matter-properties and changes answer key
Matter-properties and changes answer key Properties of matter vocabulary
Properties of matter vocabulary