Topic Properties of Liquids Do Now Properties of








- Slides: 15

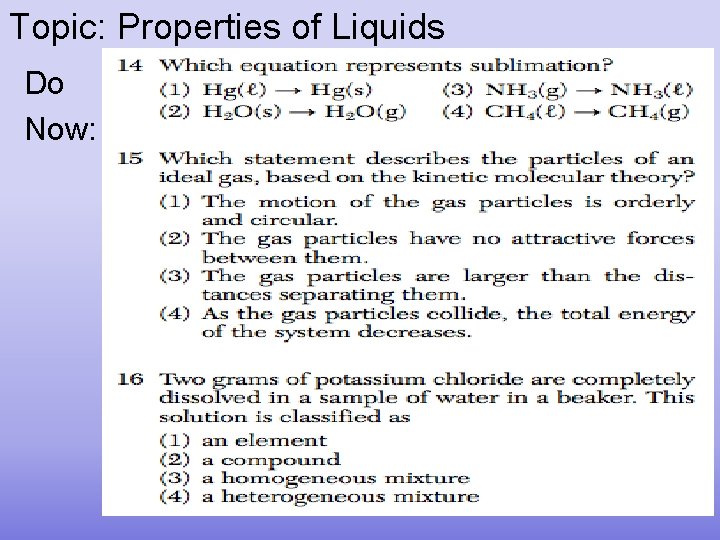
Topic: Properties of Liquids Do Now:

Properties of Liquids 1. Definite volume 2. Indefinite shape 3. Particles close together, but can move little bit 1. Liquids can flow 4. Density liquids much greater than gases 5. Liquids can be compressed but change in volume is only a small amount & requires enormous pressure 6. Have viscosity

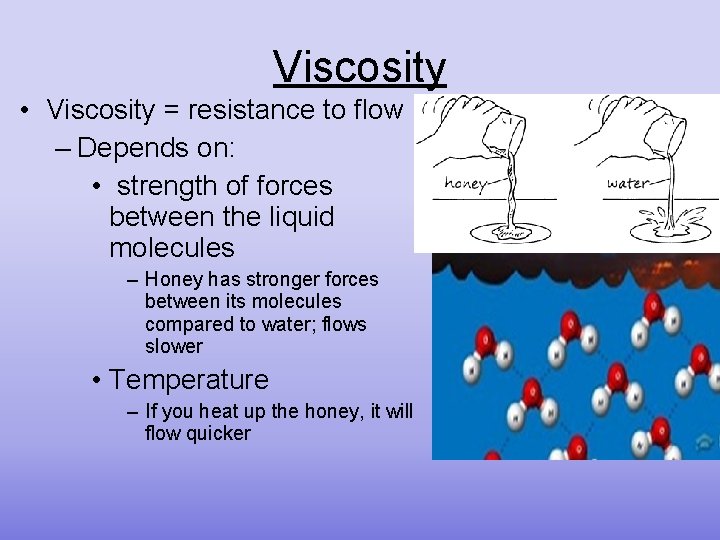
Viscosity • Viscosity = resistance to flow – Depends on: • strength of forces between the liquid molecules – Honey has stronger forces between its molecules compared to water; flows slower • Temperature – If you heat up the honey, it will flow quicker

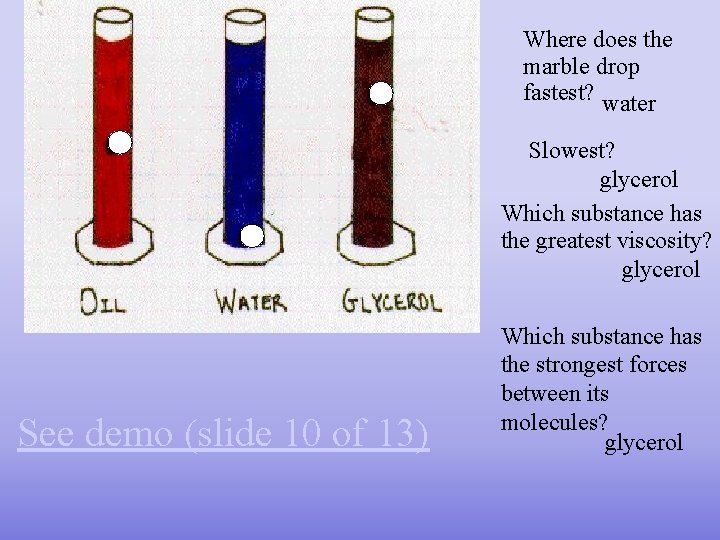
Where does the marble drop fastest? water Slowest? glycerol Which substance has the greatest viscosity? glycerol See demo (slide 10 of 13) Which substance has the strongest forces between its molecules? glycerol

FYI: ENGINE OIL • engine oil prevents direct metal to metal contact • thin film oil on surfaces prevents metal from flaking • if oil too thick, won’t circulate at low temps • if oil too thin, will lose film strength at high temps

Properties of Liquids 1. Definite volume 2. Indefinite shape 3. Particles close together, but can move little bit 1. Liquids can flow 4. Density liquids much greater than gases 5. Liquids can be compressed but change in volume is only a small amount & requires enormous pressure 6. Have viscosity 7. Surface tension

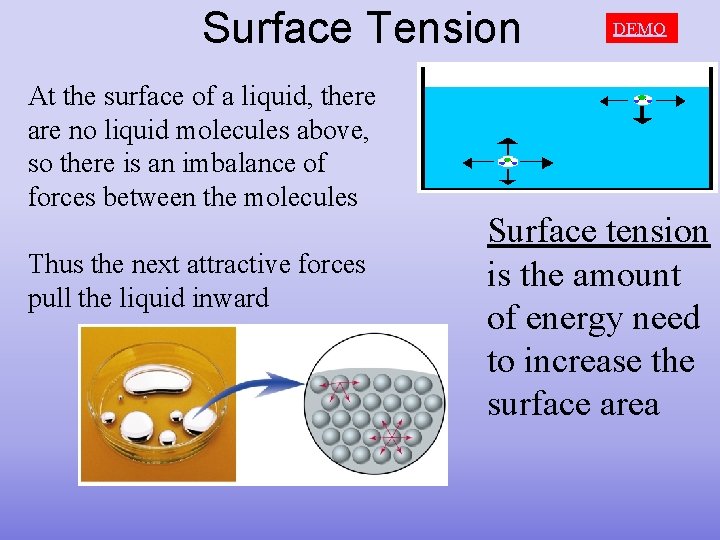
Surface Tension At the surface of a liquid, there are no liquid molecules above, so there is an imbalance of forces between the molecules Thus the next attractive forces pull the liquid inward DEMO Surface tension is the amount of energy need to increase the surface area

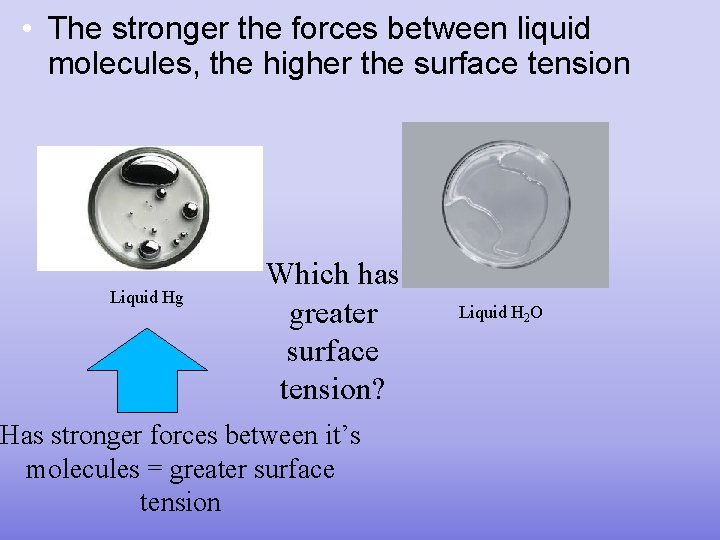
• The stronger the forces between liquid molecules, the higher the surface tension Liquid Hg Which has greater surface tension? Has stronger forces between it’s molecules = greater surface tension Liquid H 2 O


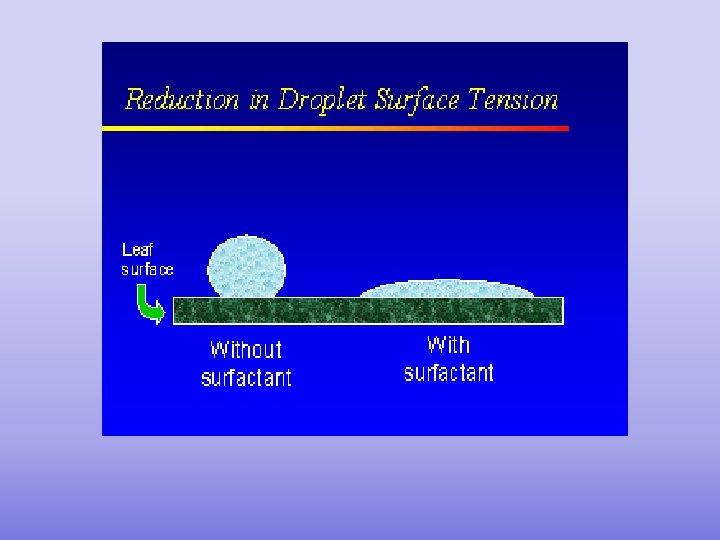
Why use Hot Water when washing dishes? DON’T • Increasing the temp. decreases the COPY surface tension – meaning water can spread out = better wetting agent • But if you use soap (it’s a surfactant, meaning it decreases the surface tension) so you don’t need the hot water


Don't touch the tent! Common tent materials are somewhat rainproof in that the surface tension of water will bridge the pores in the finely woven material. But if you touch the tent material with your finger, you break the surface tension and the rain will drip through. DON’T COPY

Properties of Liquids 1. Definite volume 2. Indefinite shape 3. Particles close together, but can move little bit 1. Liquids can flow 4. Density liquids much greater than gases 5. Liquids can be compressed but change in volume is only a small amount & requires enormous pressure 6. Have viscosity 7. Surface tension 8. Capillary Action

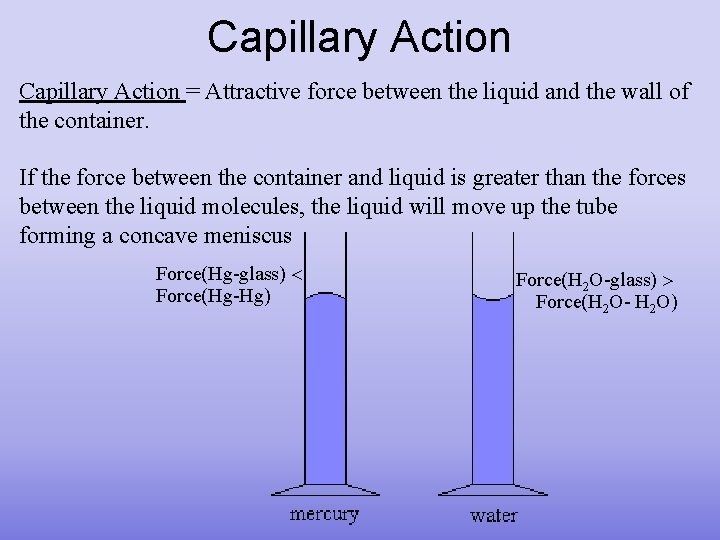
Capillary Action = Attractive force between the liquid and the wall of the container. If the force between the container and liquid is greater than the forces between the liquid molecules, the liquid will move up the tube forming a concave meniscus Force(Hg-glass) Force(Hg-Hg) Force(H 2 O-glass) Force(H 2 O- H 2 O)

 Properties of solid, liquid and gas
Properties of solid, liquid and gas Properties of liquid state of matter
Properties of liquid state of matter Liquid information
Liquid information Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Solid to gas
Solid to gas Now i see it now you don't
Now i see it now you don't Paragraph writing strategies
Paragraph writing strategies Narrowed down topic examples
Narrowed down topic examples Viscosity of liquids
Viscosity of liquids Molecular theory of gases and liquids
Molecular theory of gases and liquids Thermal expansion in liquids
Thermal expansion in liquids Do all liquids evaporate at the same rate
Do all liquids evaporate at the same rate Regional metamorphism
Regional metamorphism Kinetic molecular theory of liquids
Kinetic molecular theory of liquids What two elements are liquids at room temperature
What two elements are liquids at room temperature Example of solid
Example of solid




























