CHAPTER 2 Properties of Matter PROPERTIES OF MATTER























- Slides: 43

CHAPTER 2 Properties of Matter

PROPERTIES OF MATTER Extensive-depends on the amount of matter in a sample ex: volume, mass, texture Intensive-depends on the type of matter in a sample ex: density, viscosity, melting point

EXTENSIVE PROPERTIES It depends on the amount of matter in a sample Mass (grams) Weight (newtons) volume (m. L or cm 3)

INTENSIVE PROPERTIES Depends on type of matter Density(at STP) - boiling point texture - melting point odor - color taste - ductility hardness - solubility malleability - tenacity conductivity - brittleness

STATES OF MATTER Solid Liquids Gases 4 th state? What is plasma? 5 th state? NOVA Bose Einstein Constant



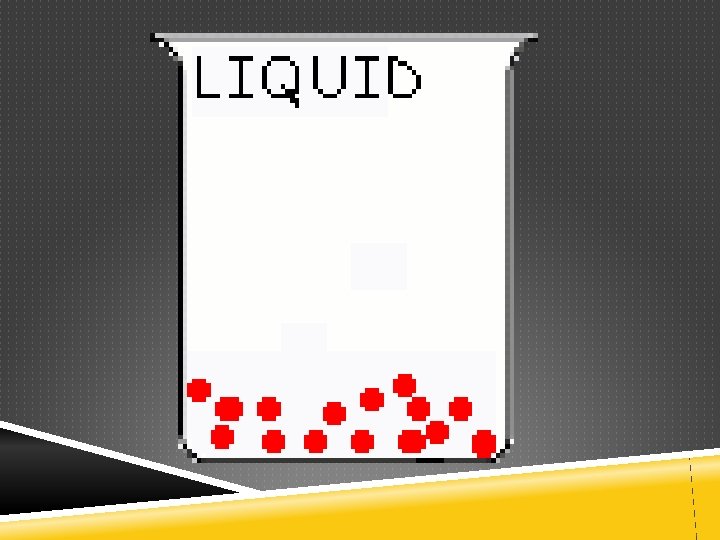
Liquids • Molecules have room to slide over each other • Have no definite shape, but have a definite volume. • Not easily compressed


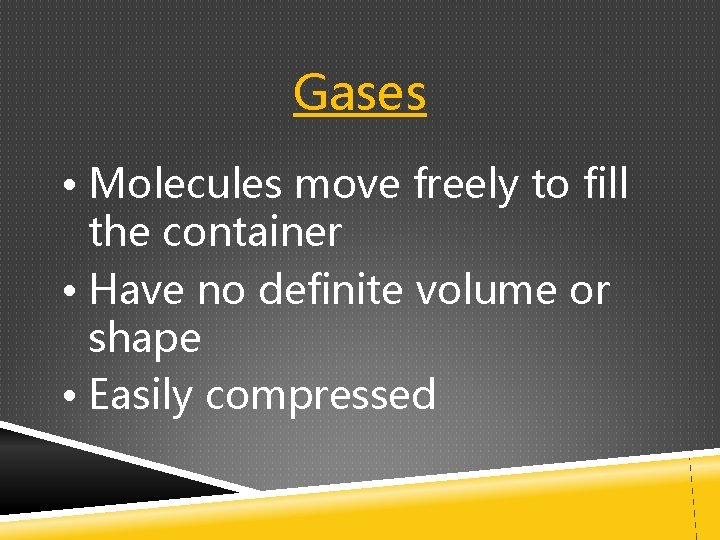
Gases • Molecules move freely to fill the container • Have no definite volume or shape • Easily compressed


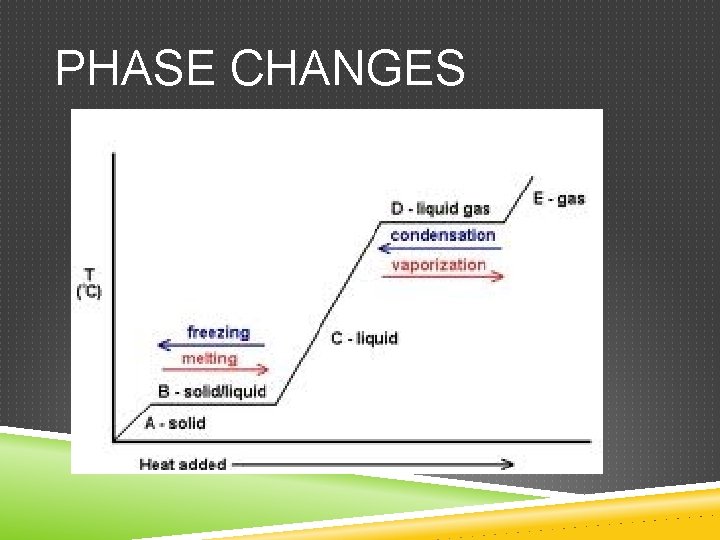
PHASE CHANGES

PHASE CHANGES AND ENERGY Exothermic- heat is released Endothermic- heat is absorbed Is the evaporation of water exothermic or endothermic? Explain.

PHASE CHANGES AND ENERGY Entropy- a measure of disorder of a system The more ordered a system is, the more entropy it has – for example a clean, organized room has less entropy than a messy room. Which has more entropy, a solid or a gas?

PHYSICAL CHANGES During a physical change, the properties of the material changes, but not the composition of the material Can be classified as reversible or irreversible Examples:

MIXTURES Mixture-physical blend of two or more components Heterogeneous ex: Homogenous (solution) ex:

ALLOYS Alloy – homogenous mixture of metals Sterling silver- 93% silver 7% copper Surgical steel- Fe – 67%, Cr 18%, Ni-12%, Mo- 3% Spring steel- Fe- 98. 6%, Cr- 1. 0%

SEPARATING MIXTURES What are some ways that we can separate mixtures?

• • • Separating mixtures by physical means Filtering By hand Boiling- fractional distillation Freezing Melting Evaporating Dissolving Crystallizing Magnetism

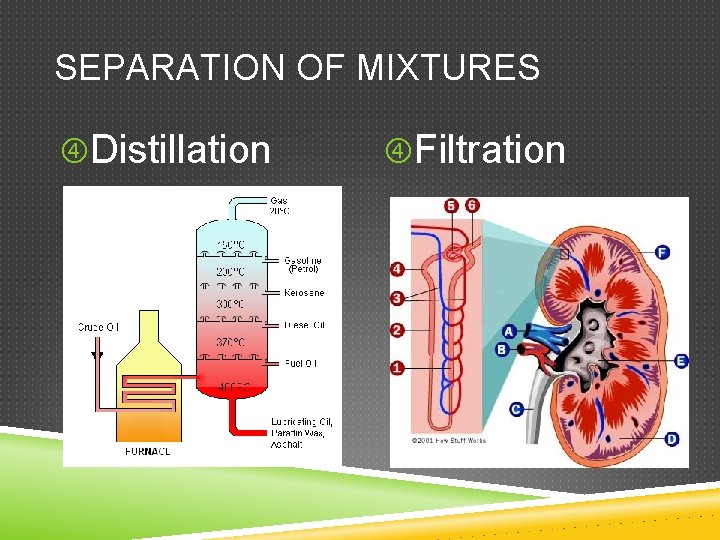
SEPARATION OF MIXTURES Distillation Filtration

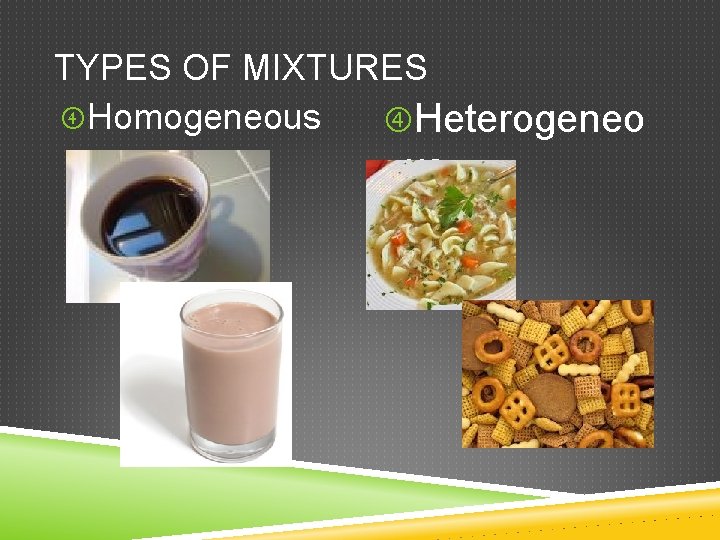
TYPES OF MIXTURES Homogeneous Heterogeneo us

ELEMENTS AND COMPOUNDS Element- simplest form of matter with a specific set of properties Compound- contains two or more elements chemically combined Compounds can be broken down into simpler substances by chemical means, elements cannot

ELEMENTS AND COMPOUNDS Elements are represented by symbols Compounds are represented by chemical formulas, groups of element symbols

ELEMENT SYMBOLS

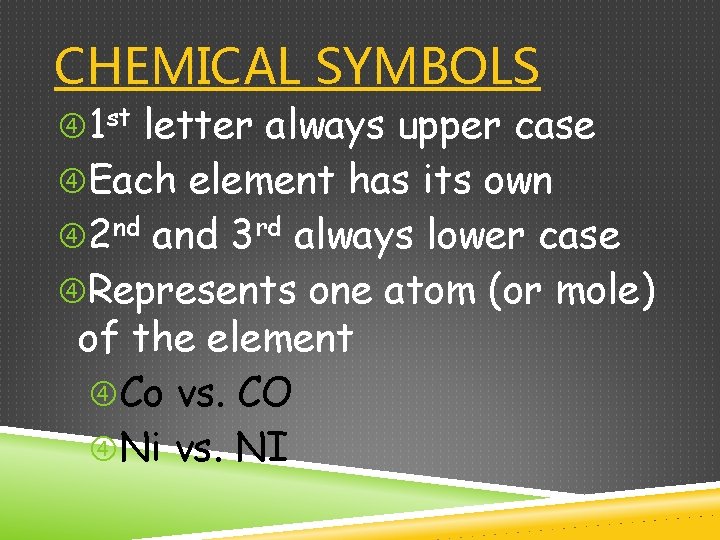
CHEMICAL SYMBOLS 1 st letter always upper case Each element has its own 2 nd and 3 rd always lower case Represents one atom (or mole) of the element Co vs. CO Ni vs. NI

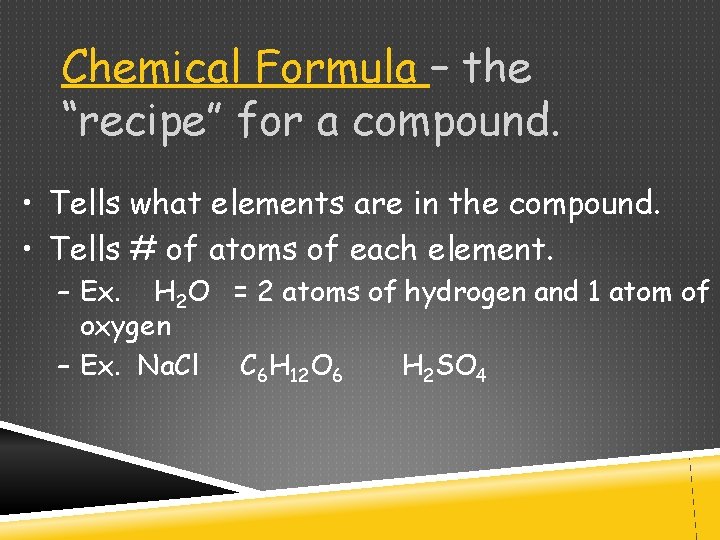
Chemical Formula – the “recipe” for a compound. • Tells what elements are in the compound. • Tells # of atoms of each element. – Ex. H 2 O = 2 atoms of hydrogen and 1 atom of oxygen – Ex. Na. Cl C 6 H 12 O 6 H 2 SO 4

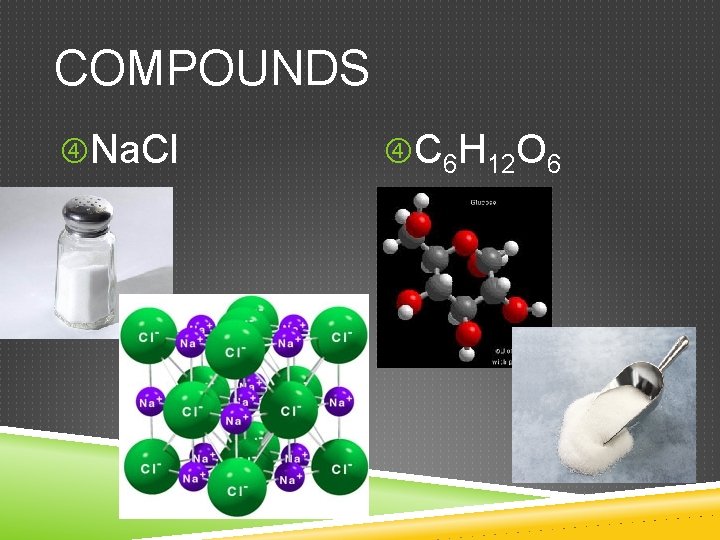
COMPOUNDS Na. Cl C 6 H 12 O 6

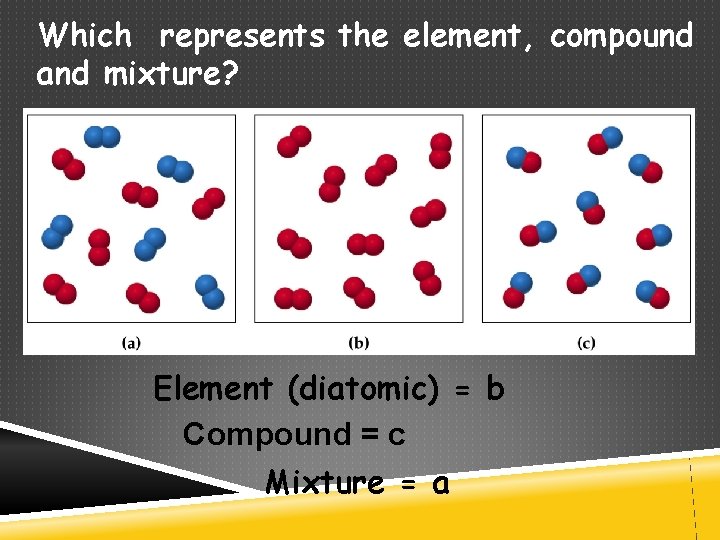
Which represents the element, compound and mixture? Element (diatomic) = b Compound = c Mixture = a

CHEMICAL CHANGES (RXN) During a chemical change, the composition of matter always changes Reactant Product

INDICATORS OF CHEMICAL CHANGE Formation of precipitate Production of a gas Change in p. H Energy change Color change Light

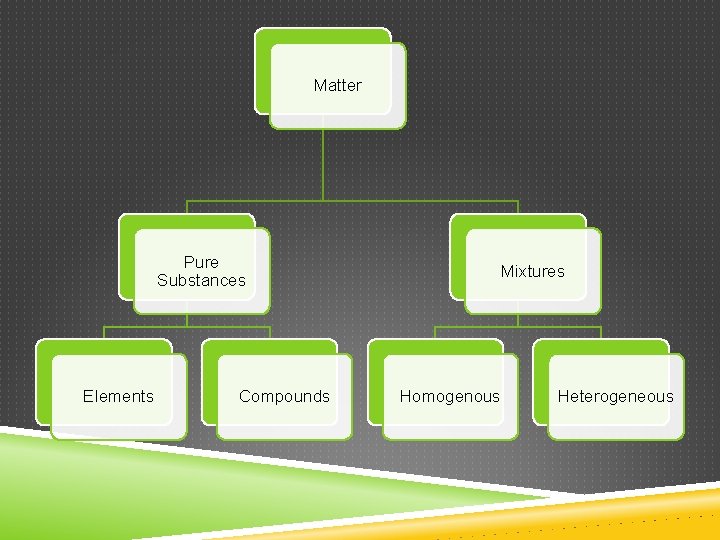
Matter Pure Substances Elements Compounds Mixtures Homogenous Heterogeneous

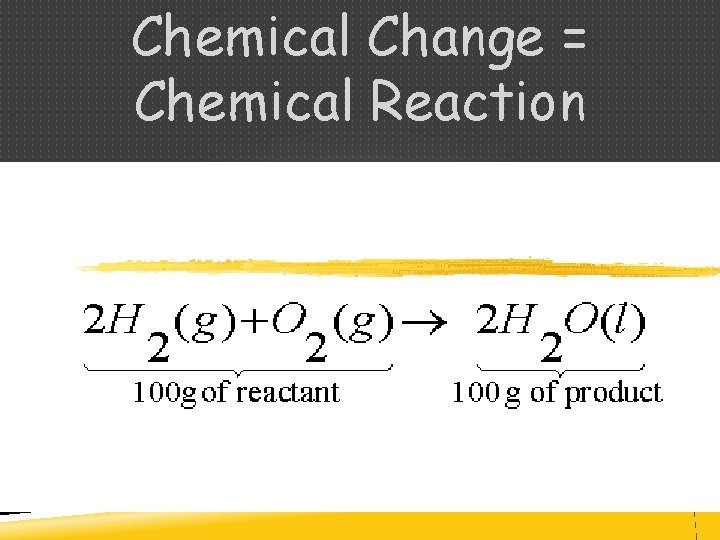
CONSERVATION OF MASS - During a chemical reaction, the mass of the products is always equal to the mass of reactants - Law of Conservation of Mass

Chemical Change = Chemical Reaction

Which of the following is not a chemical change? A. Paper being shredded B. Steel rusting C. Charcoal burning D. Newspaper yellowing in the sun

2. Which phrase best describes an apple? A. Heterogeneous mixture B. Homogenous compound C. Heterogeneous substance D. Homogenous mixture

3. Which element is paired with the wrong symbol? A. sulfur, S B. nitrogen, N C. potassium, P D. calcium, Ca

4. Which of these properties could not be used to distinguish between table salt and sugar A. Boiling point B. Melting point C. Density D. Color

5. The state of matter characterized by a definite volume and an indefinite shape is a A. Solid B. Liquid C. Mixture D. Gas

6. Which description below correctly identifies air? A. Compound B. Heterogeneous mixture C. Element D. Homogeneous mixture

7. Which description below correctly identifies carbon monoxide? A. Compound B. Heterogeneous mixture C. Element D. Homogeneous mixture

8. Which description below correctly identifies zinc? A. Compound B. Heterogeneous mixture C. Element D. Homogeneous mixture

9. Which description below correctly identifies pepperoni pizza? A. Compound B. Heterogeneous mixture C. Element D. Homogeneous mixture

10. Magnesium metal burns vigorously in oxygen to produce the compound magnesium oxide. Use the Law of Conservation of Mass and the table below to determine the mass of oxygen used in the Mass of magnesium Mass of oxygen Mass of magnesium reaction. oxide 6. 5 g ? 10. 8 g