Matter Properties and Changes Properties of Matter Changes











- Slides: 24

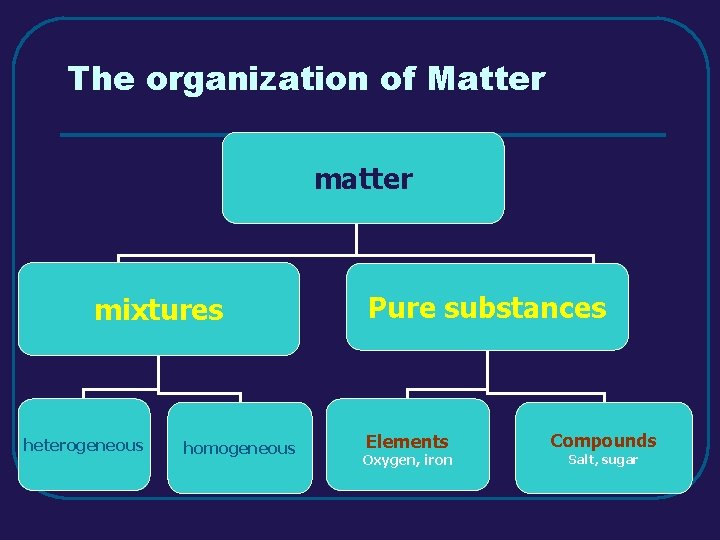
Matter – Properties and Changes Properties of Matter Changes of Matter Mixtures of Matter Elements and Compounds

PROPERTIES OF MATTER (3. 1) A. Types of Properties 1. Physical Properties - properties that can be observed and measured without changing the composition a. examples – color, texture, hardness b. types of physical properties 1) intensive - do not depend upon amount of the substance (density, mp, bp) 2) extensive - depend upon amount of the substance (mass, volume, length)

I. PROPERTIES OF MATTER(3. 1) A. Types of Properties 2. Chemical Properties - properties relating to the ability of substances to combine with or change into other substance (has a uniform and unchanging composition) a. examples 1) reaction with water 2) ability to combine with oxygen 3) reaction with acids

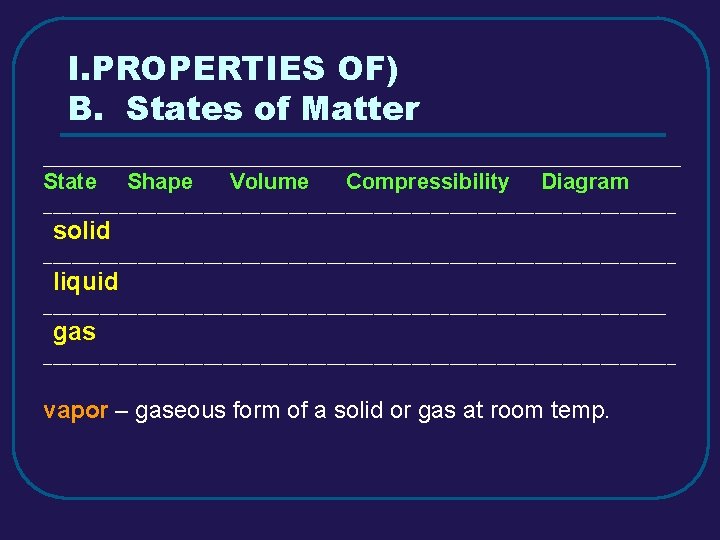
I. PROPERTIES OF) B. States of Matter _________________________________________ State Shape Volume Compressibility Diagram __________________________________ solid __________________________________ liquid _________________________________ gas __________________________________ vapor – gaseous form of a solid or gas at room temp.

II. CHANGES IN MATTER (3. 2) A. Types of Changes 1. physical - changes in which a substance is altered without changing composition a. examples- phase changes, cut into pieces 2. chemical - changes in which one or more substances change into new substances with new properties a. reactants – substances that react b. products - substances that are produced

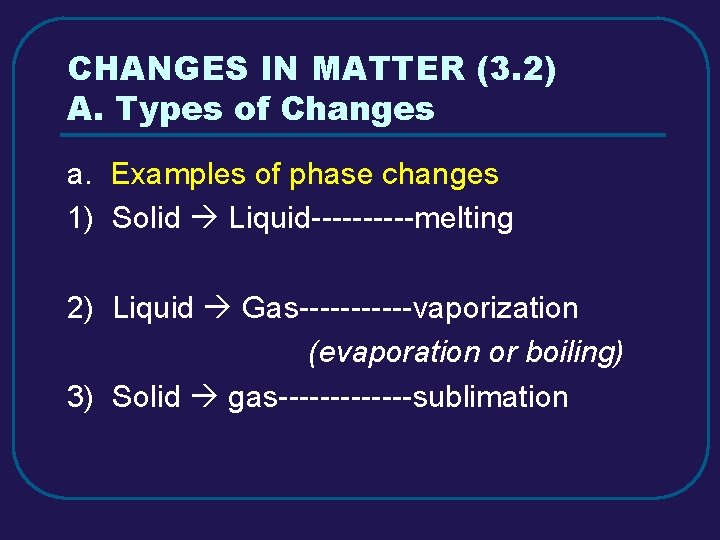
CHANGES IN MATTER (3. 2) A. Types of Changes a. Examples of phase changes 1) Solid Liquid-----melting 2) Liquid Gas------vaporization (evaporation or boiling) 3) Solid gas-------sublimation

CHANGES IN MATTER (3. 2) A. Types of Changes (Physical) a. Examples of phase changes 4)Gas Liquid ----- Condensation 5)Liquid Solid ----- Solidification (freezing) 6)Gas Solid ------- Deposition

2. Chemical changes c. Examples of chemical changes 1) digestion of food 2) burning (combustion) 3) fermentation 4) rusting 5) tarnishing of silver

II. CHANGES IN MATTER (3. 2) B. Evidence of Chemical Changes 1. change in color and/or appearance 2. change in energy 3. change in odor 4. formation of a precipitate or a gas

III. MIXTURES (3. 3) A. Definition of Mixture - combination of pure substances that retain their properties B. Types of Mixtures 1. heterogeneous - individual substances are distinct (composition is not uniform throughout) a. examples – sand, vegetable soup 2. homogeneous - individual substances are not distinct (uniform composition throughout) a. examples - solutions

III. MIXTURES (3. 3) B. Types – 2. homogeneous _____________________________________________________ type of solution examples _____________________________________________________ solid – liquid sugar water, salt water ______________________ gas – liquid carbonated beverages ____________________________ liquid – liquid alcohol, vinegar ____________________________ gas – gas air ____________________________ solid - solid alloys (metal + metal) ____________________________

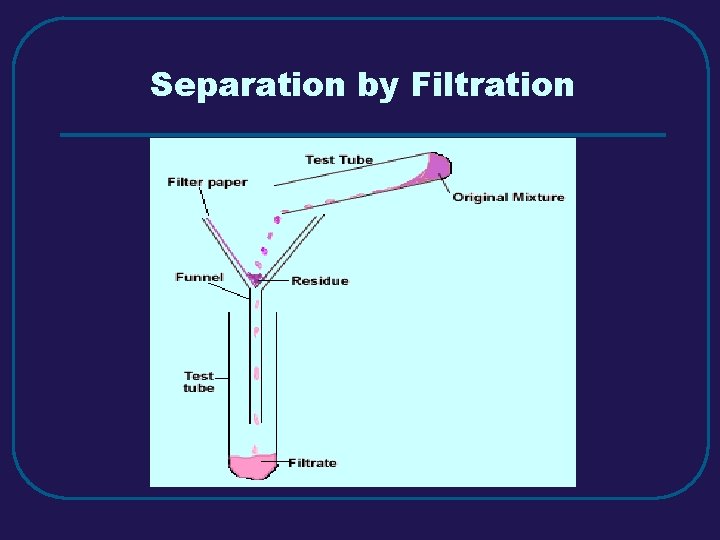
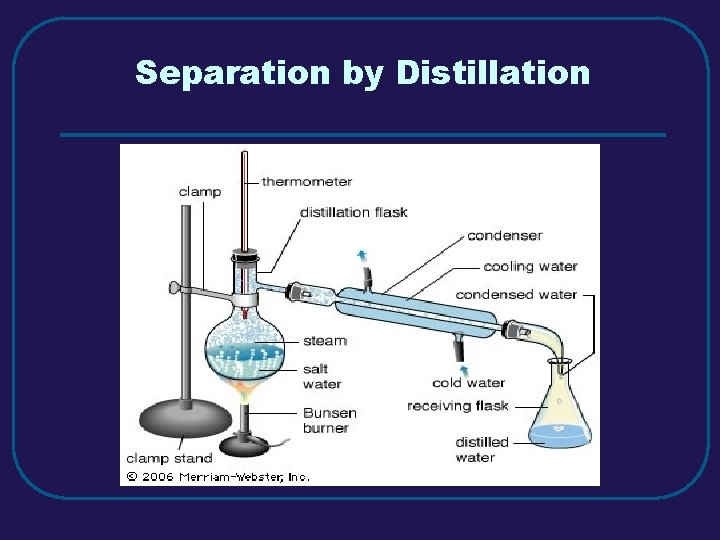
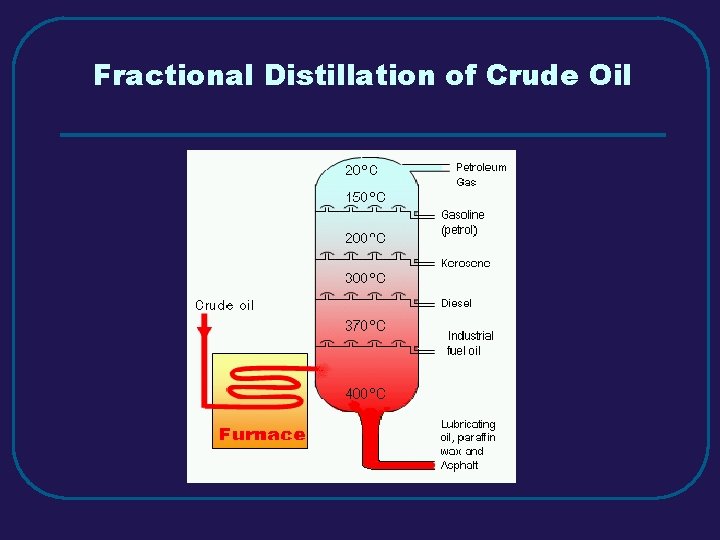
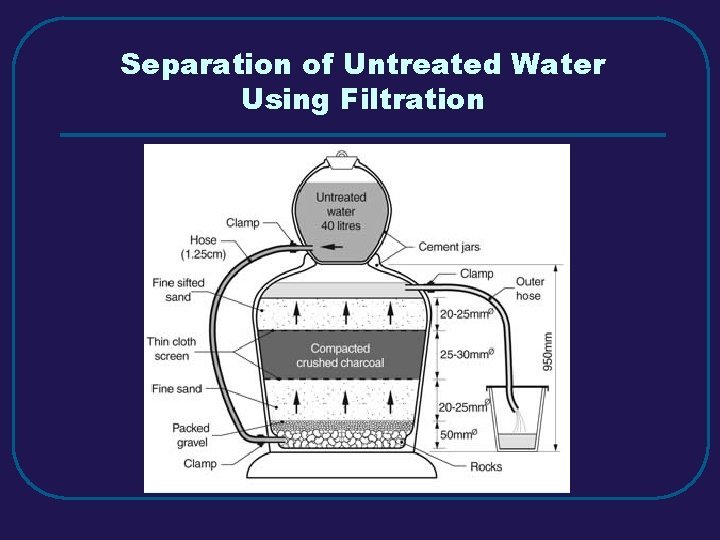
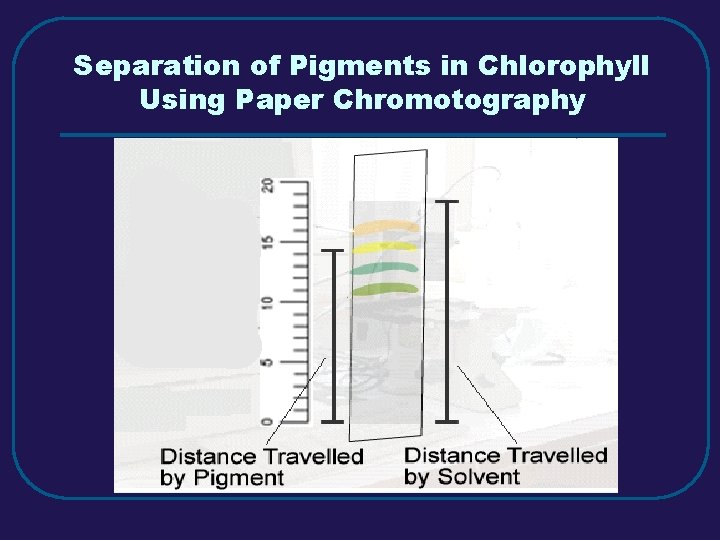
III. MIXTURES (3. 3) C. Separating Mixtures 1. Filtration – separate solid from a liquid in a heterogeneous mixture 2. Distillation – separate homogeneous mixtures – based on differences in boiling points 3. Crystallization – separates a homogeneous mixture by causing crystals to form 4. Chromatography – separation of components of a solution (mobile phase) based on tendency to move on the stationary phase

Decantation

Separation by Filtration

Separation by Distillation

Fractional Distillation of Crude Oil

Distillation of Oil

Separation of Untreated Water Using Filtration

Separation of Black Ink Using Paper Chromatography

Separation of Pigments in Chlorophyll Using Paper Chromotography

Separation by Crystallization

IV. ELEMENTS AND COMPOUNDS(3. 4) A. Elements 1. definition - elements are pure substances that can not be separated into simpler substances by physical or chemical means 2. examples and their symbols (C, N, Na, Co) 3. periodic table of elements a. periods – horizontal rows b. groups (families) – vertical columns 1) similar structure and properties

IV. ELEMENTS AND COMPOUNDS (3. 4) 1. definition - a compound is a pure substance that is the combination of two or more different elements 2. examples of compounds a. sodium bicarbonate Na. HCO 3 b. hydrochloric acid HCl c. sulfur dioxide SO 2

The organization of Matter mixtures heterogeneous homogeneous Pure substances Elements Oxygen, iron Compounds Salt, sugar