Properties of Matter Composition and Properties of Matter
















- Slides: 19

Properties of Matter

Composition and Properties of Matter n Composition of Matter n what matter is made of n n Ex: Center of the Earth Properties of Matter n what the matter is like; how matter behaves. n Ex: Earthquakes

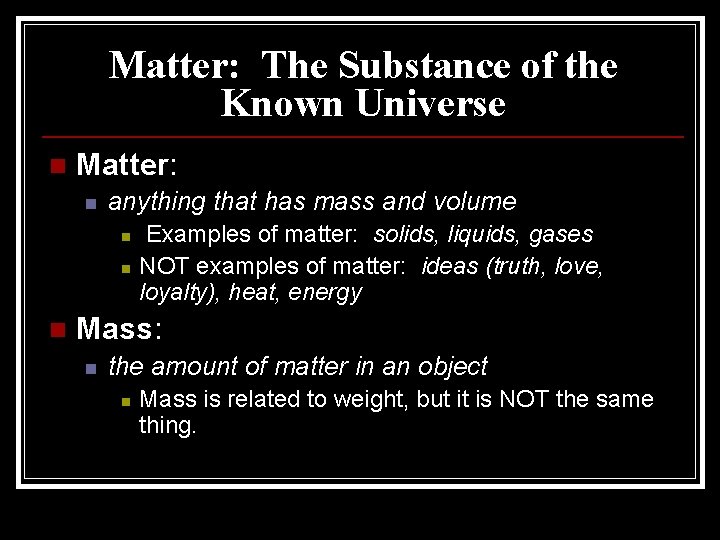
Matter: The Substance of the Known Universe n Matter: n anything that has mass and volume n n n Examples of matter: solids, liquids, gases NOT examples of matter: ideas (truth, love, loyalty), heat, energy Mass: n the amount of matter in an object n Mass is related to weight, but it is NOT the same thing.

Mass Vs. Weight n What does an object’s weight depend on? n how hard gravity pulls on it n n this will vary, depending on location An object’s mass does NOT change, no matter where it is.

Volume n the amount of space an object occupies n Is air matter? § Air takes up space; has mass

Simplest form of matter n The most basic unit of matter is the atom. There are 109 different varieties of atom. n they are listed on the Periodic Table n n Ex: oxygen (O), carbon (C), hydrogen (H) Often, atoms combine together to form a molecule n a neutral group of atoms held together by chemical bonds n Ex; Carbon dioxide, water, hydrogen molecule

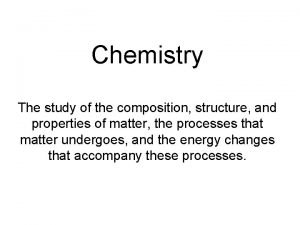
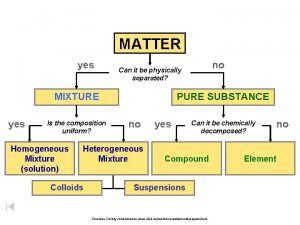
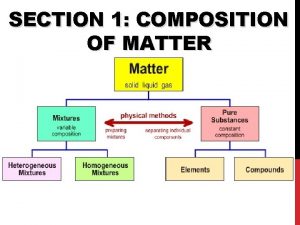
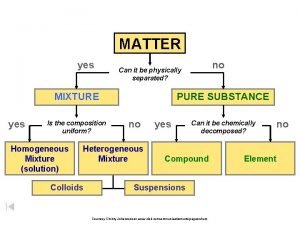
How Do We Classify Matter? At the smallest level, all matter is composed of atoms. n All matter can be classified as either a pure substance or a mixture. n n n Ex: Pure Substance; pure gold (24 karat) Ex: Mixture; gold alloy (14 karat)

Pure Substance a type of matter for which all samples have the same properties; they behave exactly the same way n There are 2 types of pure substances, elements and compounds n

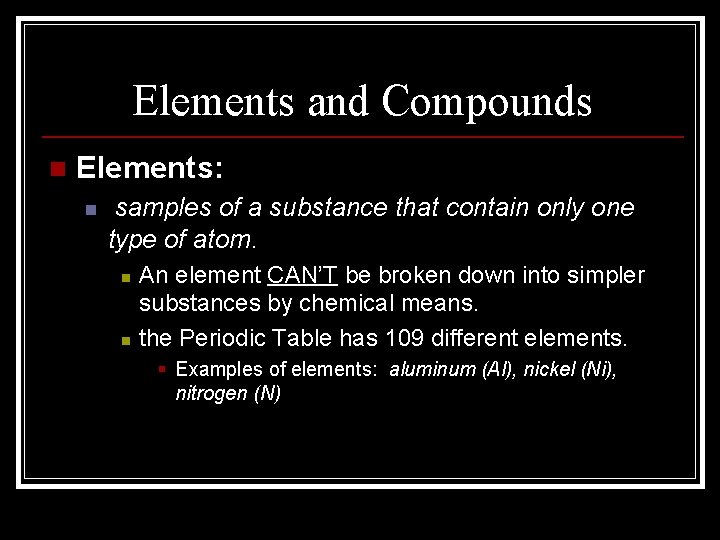
Elements and Compounds n Elements: n samples of a substance that contain only one type of atom. n n An element CAN’T be broken down into simpler substances by chemical means. the Periodic Table has 109 different elements. § Examples of elements: aluminum (Al), nickel (Ni), nitrogen (N)

Elements and Compounds Cont. n Allotropes: n different forms of an element in the same physical state n n Ex: oxygen atom, oxygen gas, ozone O O 2 O 3 Compounds: n a substance made up of 2 or more different elements that are chemically combined n Methane (CH 4), Water (H 2 O)

Compounds Cont. n Properties of Compounds: n n every sample of a particular compound has the same properties as every other sample Sample problem: n In every 100 g sample of pure water, there are 11. 2 g of hydrogen and 88. 8 g of oxygen. How many grams of hydrogen are in a 120 g sample of pure water?

Mixtures n Mixtures: n n n a combo of 2 or more substances; each retains its individual properties In a mixture, there are no chemical bonds between the different substances. There are two types of mixtures n n homogeneous mixture heterogeneous mixture

Homogeneous mixture n all regions of a homogeneous mixture are identical in composition and properties n n Homogeneous mixtures are evenly-mixed, or uniformly distributed, at the particle level, and are also referred to as solutions. Ex: soda pop, salt water, sugar water, Kool-Aid n alloy = a homogeneous mixture of 2 or more metals § Ex: brass = copper + zinc,

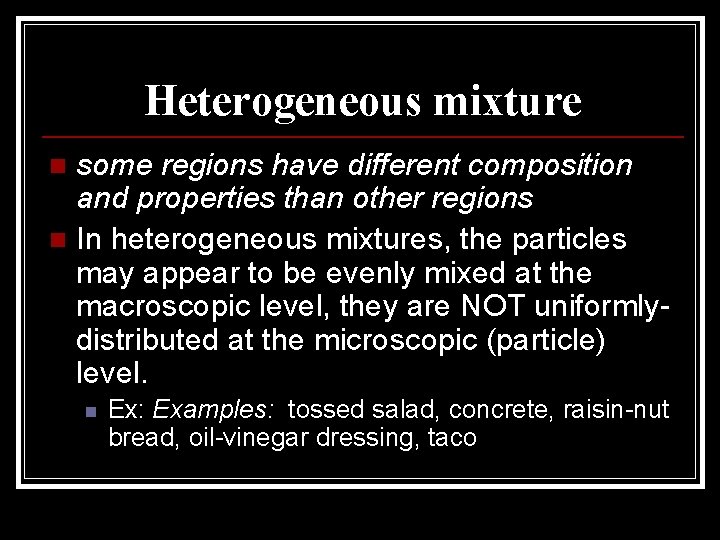
Heterogeneous mixture some regions have different composition and properties than other regions n In heterogeneous mixtures, the particles may appear to be evenly mixed at the macroscopic level, they are NOT uniformlydistributed at the microscopic (particle) level. n n Ex: Examples: tossed salad, concrete, raisin-nut bread, oil-vinegar dressing, taco

Heterogeneous mixture n Other types of Heterogeneous Mixtures n suspension = appears uniform while stirred; settles when agitation stops n n Examples: Quick milk, muddy water, OJ with pulp, oil & vinegar dressing colloid = contains tiny particles that never settle out n Examples: gelatin, milk, smoke, fog

Characteristics that Distinguish Pure Substances from Mixtures n 1. A pure substance has only one set of properties, but a mixture retains the properties of each of its constituents. n 2. The composition of a pure substance is fixed, but the composition of a mixture can vary widely.

Physical and Chemical properties and changes n n n All matter exhibits physical and chemical properties Physical properties: color, odor, density, hardness, solubility, melting point & boiling point. Chemical properties: are those exhibited when a substance reacts w/ other substances. n Ex: reactions with acids and bases and other chemicals

Physical Change n n n n The altering of shape, size, or physical state but chemical composition stays the same. Solid Liquid Melting Gas Liquid Condensation Solid Gas Sublimation Liquid Solid Freezing or Crystallizing Gas Solid Reverse Sublimation Liquid Gas Boiling or Evaporation

Chemical Change n The atoms of a substance are rearranged n A new substance(s) formed has a chemical composition that is different than the original substance(s)
 The study of composition structure and properties of matter
The study of composition structure and properties of matter Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is a uniform composition
What is a uniform composition Properties of solids liquids and gases
Properties of solids liquids and gases Composition of matter which depends on temperature
Composition of matter which depends on temperature Composition of matter section 1
Composition of matter section 1 Variable composition
Variable composition States of matter flow chart
States of matter flow chart Composition of matter chapter 9
Composition of matter chapter 9 Section 1 composition of matter
Section 1 composition of matter Matter concept map
Matter concept map Composition of matter flow chart
Composition of matter flow chart Composition of matter notes
Composition of matter notes Mixture
Mixture Meysam golmohammadi
Meysam golmohammadi Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon