Chemistry The study of the composition structure and


















- Slides: 27

Chemistry The study of the composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany these processes.

Branches of Chemistry • Organic Chemistry – The study of carbon containing compounds • Inorganic Chemistry – The study of non-organic substances • Physical Chemistry – The study of the properties and changes of matter and their relation to energy • Analytical Chemistry – The identification of the components and composition of materials • Biochemistry – The study of substances and processes occurring in living things • Theoretical Chemistry – The study of what’s next in chemistry

Why we need to DO Chemistry • Basic Research – Basic research in chemistry allows us to explain why things happen in nature • Applied research – Used to solve a problem, initiates the development of new concepts and materials • Technological Development – Involves the production and use of new products that improve our quality of life (others quality of life too, sometimes)

Scientific Method • Step 1) State/Identify the Problem • Step 2) Observations – Use your senses to collect any and all information about the problem – May be qualitative (descriptive) – May be quantitative (numerical) – Isolate your system • Surroundings specifically dealing with the problem

• Step 3) Formulate a Hypothesis – Hypothesis is a possible solution to the problem – Can be formulated as an “if-then” type statement • Step 4) Test hypothesis – This is an experiment portion of the scientific method – Independent variable is used to compare “experimental” variable against. Typically set up before the experiment starts – Dependent variable is the variable you are testing – Controls are used to isolate all other variables other than the dependent or testing variables

• Step 4) Theorizing – Make conclusions about your experiment and hypothesis – Models can be used as visual representations of your conclusion • Step 5) Modify (if necessary) – Make changes to your hypothesis based on your conclusions. – Repeat the whole process if necessary

Matter and Its Properties • Matter is anything that has mass and takes up space. – Mass is a measure of the amount of matter in an object – Volume is the amount of space matter occupies

Building Blocks of Matter • The Atom is the smallest unit of matter that maintains its own physical and chemical properties – Protons, electrons, and neutrons are all the same for every type of atom. They are obviously smaller than an atom but do not maintain their own unique properties. • An Element is a pure substance that cannot be broken down into simpler substances. – Made up of only one type of atom • Compounds are made up of more than one atom, chemically bonded together, thus are able to be broken down into simpler substances

Properties and Changes in Matter • A property is a characteristic that defines an entire set of substances. – Properties are used to classify matter into groups, making it easier to identify unkown substances • Extensive properties depend on the amount of matter that is present – Volume, mass, energy • Intensive properties do not depend on the amount of matter present – Melting point, boiling point, density, conductivity

Physical properties • Physical properties are characteristics that can be observed without changing the identity of the substance. – Describes itself and not changes it undergoes – Melting point, boiling point, physical state, color, shape, etc. • Physical changes involve a change in a physical property as long as no NEW substance is created. – Changes of state are PHYSICAL CHANGES

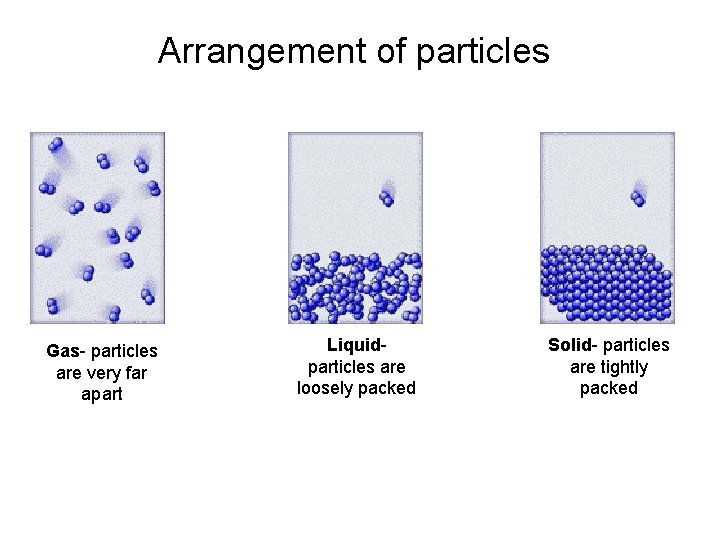
States of matter • Solids have a definite shape and definite volume. • Particles are tightly packed together almost in a fixed vibrating position – Crystalline solids have pretty geometric patterns • They have definite melting points • The particles which make them up form slowing over time allowing them to arrange themselves into geometric patterns – Non-Crystalline solids are NOT crystalline solids • They have all the properties that crystalline solids don’t have

States of Matter cont. • Liquids have a definite volume but no definite shape • Particles are loosely packed together – Particles are loose enough to move around each other allowing liquids to “flow”

States of Matter cont. • Gases have no definite volume and no definite shape – Completely fill whatever container they are in • Particles are very far apart – Particles undergo “perfectly elastic collisions” when they do come into contact with each or the container walls • The particles maintain all the energy they had before any collision

Arrangement of particles Gas- particles are very far apart Liquidparticles are loosely packed Solid- particles are tightly packed

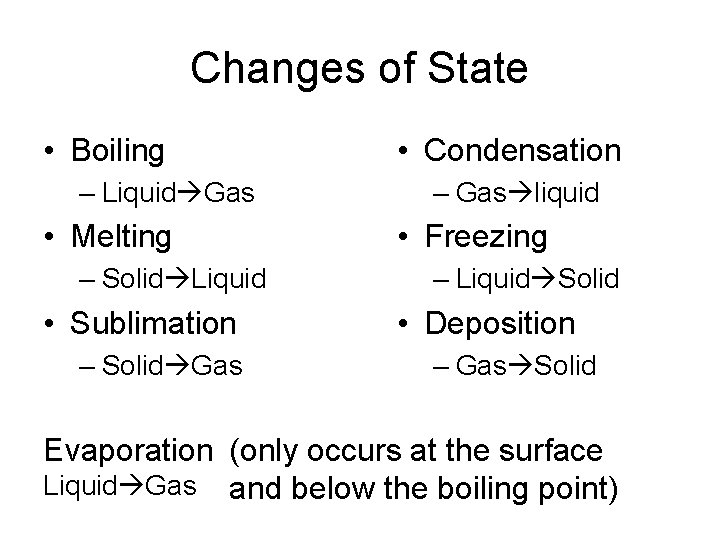
Changes of State • Boiling – Liquid Gas • Melting – Solid Liquid • Sublimation – Solid Gas • Condensation – Gas liquid • Freezing – Liquid Solid • Deposition – Gas Solid Evaporation (only occurs at the surface Liquid Gas and below the boiling point)

Chemical Properties • Chemical property relates to a substance’s ability to undergo changes that transform it into different substances. – Something new is made and the original substance is NOT easily recovered – Burning, rusting, tarnishing, oxidizing

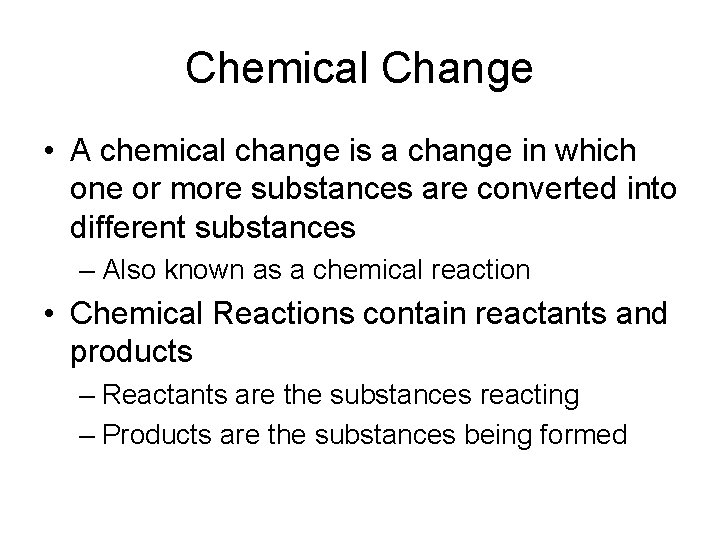
Chemical Change • A chemical change is a change in which one or more substances are converted into different substances – Also known as a chemical reaction • Chemical Reactions contain reactants and products – Reactants are the substances reacting – Products are the substances being formed

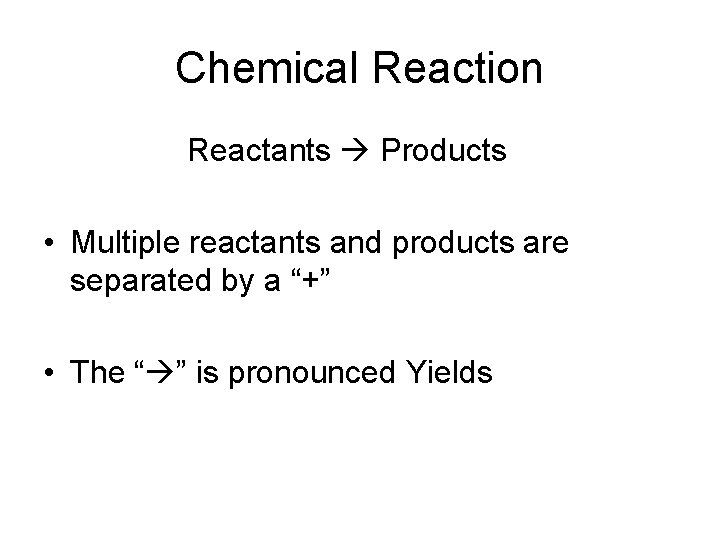
Chemical Reaction Reactants Products • Multiple reactants and products are separated by a “+” • The “ ” is pronounced Yields

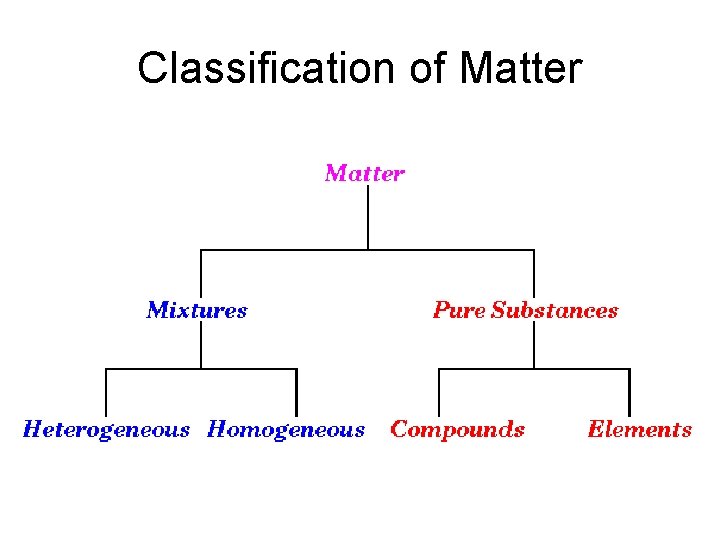
Classification of Matter

Mixture • Two or more substances, combined in varying proportions - each retaining its own specific properties. • The components of a mixture can be separated by physical means, i. e. without the making and breaking of chemical bonds. • Examples: Air, salt water, milk, wood, and concrete.

Mixtures cont. • Heterogeneous Mixture – Mixture in which the properties and composition are not uniform throughout the sample. – Every spoonful could be different – Examples: Milk, wood, blood, granite and concrete. • Homogeneous Mixture – Mixture in which the properties and composition are uniform throughout the sample. – Every spoonful is identical – The “best mixed” mixtures are termed solutions. – Examples: Air, stainless steel and salt water.

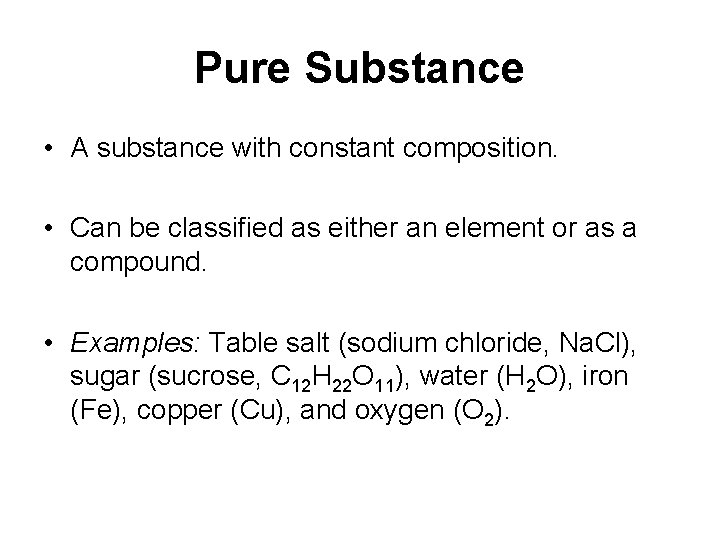
Pure Substance • A substance with constant composition. • Can be classified as either an element or as a compound. • Examples: Table salt (sodium chloride, Na. Cl), sugar (sucrose, C 12 H 22 O 11), water (H 2 O), iron (Fe), copper (Cu), and oxygen (O 2).

Compound • A substance that contains two or more elements, in definite proportion by weight. • The composition of a pure compound will be invariant, regardless of the method of preparation. • Compounds are composed of more than one kind of atom. • The term molecule is often used for the smallest unit of a compound that still retains all of the properties of the compound. • Examples: Table salt (sodium chloride, Na. Cl), sugar (sucrose, C 12 H 22 O 11), and water (H 2 O).

Element • A substance that cannot be separated into two or more substances by ordinary chemical (or physical) means. – We use the term ordinary chemical means to exclude nuclear reactions. • Elements are composed of only one kind of atom. • Examples: Iron (Fe), copper (Cu), and oxygen (O 2).

The Periodic Table • Dmitri Mendeleev, father of the modern periodic table – Born in Siberia, the last of at least 14 children, Dmitri Mendeleev revolutionized our understanding of the properties of atoms and created a table that probably adorns every chemistry classroom in the world. After his father went blind and could no longer support the family, Mendeleev’s mother started a glass factory to help make ends meet. But just as Mendeleev was finishing high school, his father died and the glass factory burned down. With most of her other children now out on their own, his mother took her son to St. Petersburg, working tirelessly and successfully to get him into college.


Mendeleev’s table as published in 1869, with many gaps and uncertainties Mendeleev’s first sketch of a periodic table of the elements