MATTER Classification Properties and Changes CLASSIFICATION ALL MATTER


















- Slides: 25

MATTER Classification, Properties and Changes

CLASSIFICATION ö ALL MATTER CAN BE CLASSIFIED AS EITHER: ØHETEROGENEOUS (TWO OR MORE PHASES) ØHOMOGENEOUS (ONE PHASE)

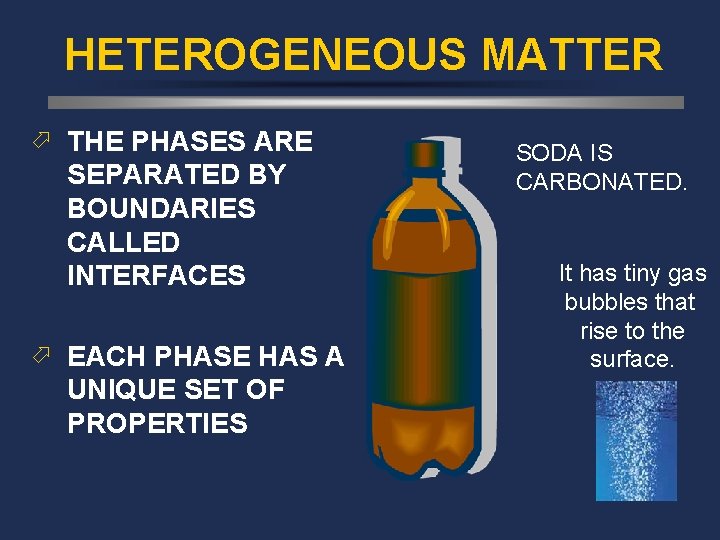
HETEROGENEOUS MATTER ö THE PHASES ARE SEPARATED BY BOUNDARIES CALLED INTERFACES ö EACH PHASE HAS A UNIQUE SET OF PROPERTIES SODA IS CARBONATED. It has tiny gas bubbles that rise to the surface.

HOMOGENEOUS MATTER ö ONE PHASE MATERIALS THAT CAN BE COMPOSED OF ONE OR MORE TYPES OF PARTICLES ö IS EITHER A SOLUTION OR PURE SUBSTANCE

SOLUTIONS ö ARE MADE OF ØA SOLVENT (DOES THE DISSOLVING) EXAMPLE: WATER ØA SOLUTE (GETS DISSOLVED) EXAMPLE: SALT THE RESULTING SOLUTION IS SALT WATER.

PURE SUBSTANCES ö CONSIST OF ONE TYPE OF PARTICLE ö ARE EITHER ØCOMPOUNDS (ONE TYPE OF MOLECULE) EXAMPLE: Na. Cl ØELEMENTS (ONE TYPE OF ATOM) EXAMPLE: Fe

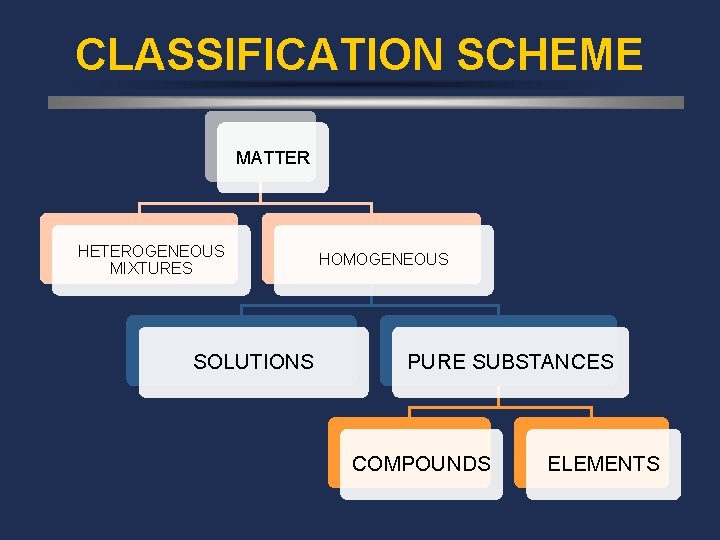
CLASSIFICATION SCHEME MATTER HETEROGENEOUS MIXTURES SOLUTIONS HOMOGENEOUS PURE SUBSTANCES COMPOUNDS ELEMENTS

PROPERTIES ö IDENTIFYING CHARACTERISTICS OF A MATERIAL ö CAN BE DETERMINED BY MEASURING AND OBSERVING THE MATERIAL UNDER DIFFERENT CONDITIONS

Physical Properties ö Physical Property w can be observed without changing the identity of the substance w Examples: w Can be intensive or extensive

PHYSICAL PROPERTIES INTENSIVE ö Do not depend upon the amount of matter present in the sample ö Examples: EXTENSIVE ö Do depend upon the amount of matter present in the sample ö Examples:

Chemical Properties ö Chemical Property w describes the ability of a substance to change its identity w Examples:

Physical vs. Chemical ö Examples: w melting point _____ w flammable _____ w density _____ w magnetic _____ w tarnishes in air _____

Physical vs Chemical ö State of Matter ö ö ö _____ Reactivity with Acid _____ Nitroglycerine explodes _____ Color _____ Food Digests _____ Volume _____ Mass _____

CHANGES ö Physical Change w changes the form of a substance without changing its identity w properties remain the same w Examples:

Types of Physical Changes ö Mechanical Changes: tearing, crushing, breaking ö Making a Solution: dissolving salt in water does not form new substances

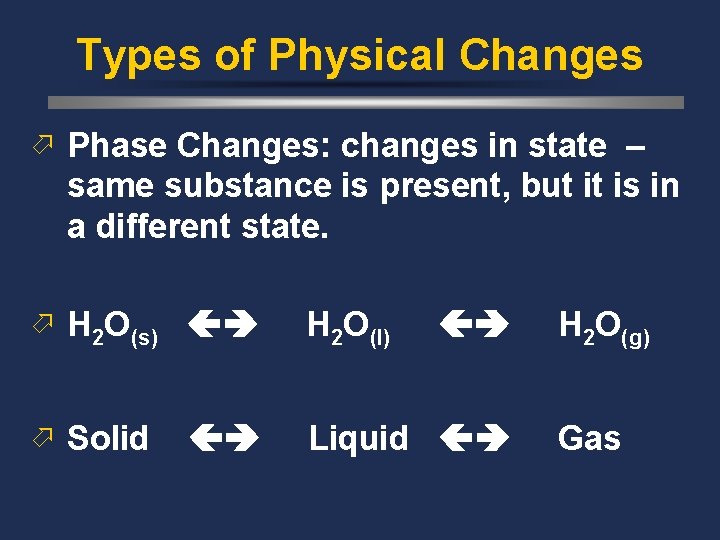
Types of Physical Changes ö Phase Changes: changes in state – same substance is present, but it is in a different state. ö H 2 O(s) H 2 O(l) ö Solid Liquid H 2 O(g) Gas

CLASSZONE ANIMATION GO TO classzone. com Animations: Chapter 8: Dissociation of a solute in a liquid Chapter 14: Change of state

CHANGES: CHEMICAL ö Chemical Change w changes the identity of a substance w products have different properties w commonly called chemical reaction w Examples: Na + Cl 2 Na. Cl Reactants produce Products

Chemical Changes ö Signs of a Chemical Change: usually a combination of these 1. Color Change 2. Formation of a gas 3. Formation of a precipitate (solid) 4. Light or Heat Release or Absorbed 5. Formation of an Odor

Physical vs. Chemical ö Examples: w rusting iron ____ w dissolving in water ____ w burning a log ____ w melting ice ____ w grinding spices ____

Chemical or Physical ? ? ? ö Mg + O 2 ö Chemical or Physical ? ö Sign or Indication? ö Equations must always be balanced: Mass is conserved (does not change) in ordinary reactions. (Lavoisier) Matter is neither created nor destroyed in ordinary chemical reactions.

CLASSZONE VIDEO GO TO classzone. com Show video: Zinc and Iodine reaction White phosphorus combustion

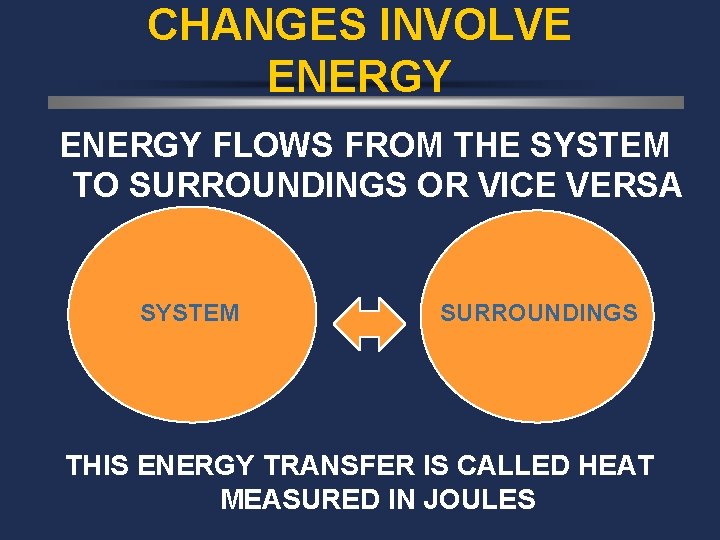
CHANGES INVOLVE ENERGY FLOWS FROM THE SYSTEM TO SURROUNDINGS OR VICE VERSA SYSTEM SURROUNDINGS THIS ENERGY TRANSFER IS CALLED HEAT MEASURED IN JOULES

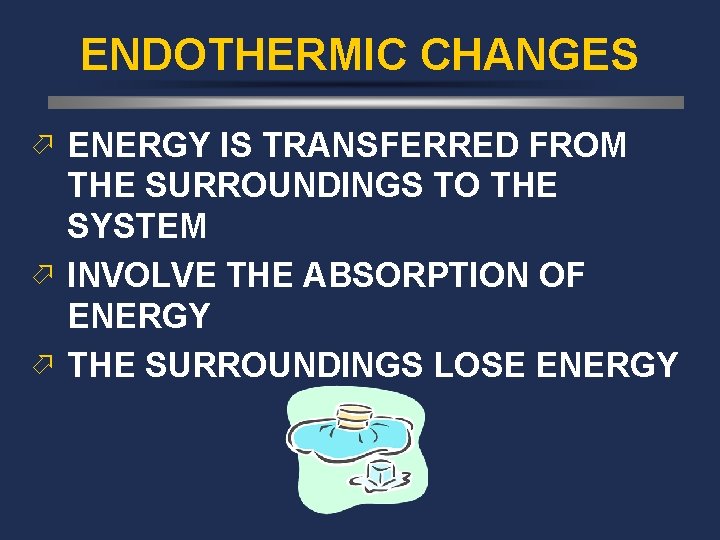
ENDOTHERMIC CHANGES ö ENERGY IS TRANSFERRED FROM THE SURROUNDINGS TO THE SYSTEM ö INVOLVE THE ABSORPTION OF ENERGY ö THE SURROUNDINGS LOSE ENERGY

EXOTHERMIC CHANGES ö ENERGY IS TRANSFERRED FROM THE SYSTEM TO THE SURROUNDINGS ö INVOLVE THE PRODUCTION OF ENERGY ö THE SURROUNDINGS GAIN ENERGY