Properties of Matter Matter All matter has two



















- Slides: 24

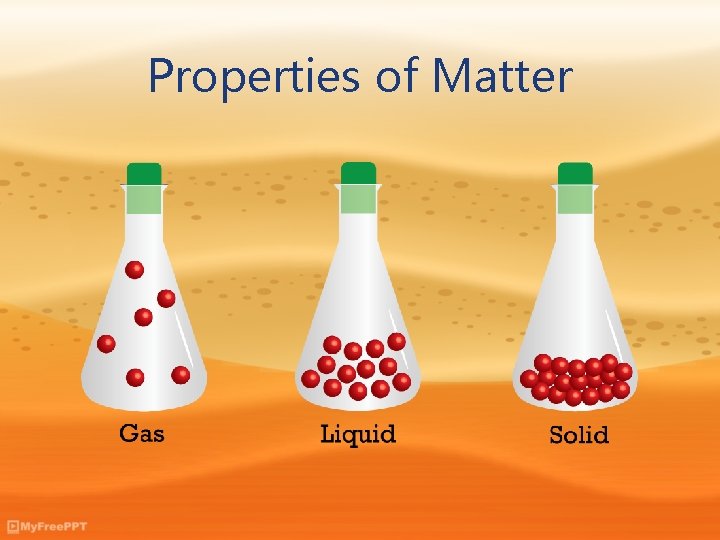
Properties of Matter

Matter • All matter has two types of properties: 1. Physical properties 2. Chemical properties

Physical properties • A physical property is a characteristic of a substance that can be observed without changing the substance into another substance. – (You can see it without changing what you’re looking at into something else. )

Physical Properties - Examples • Examples of extensive physical properties include: – Volume – Mass – Weight – Size

Physical Properties - Examples • Examples of intensive physical properties include: – Density – Melting point – Boiling point

Physical Properties - Examples • Other physical properties include: – Color – Hardness – Odor – Taste – State of matter – Texture – Luster (shine) – Flexibility – Heat conductivity – Electrical conductivity – Solubility (ability to dissolve in water. ) – Shape – Viscosity – Ductility – Malleability

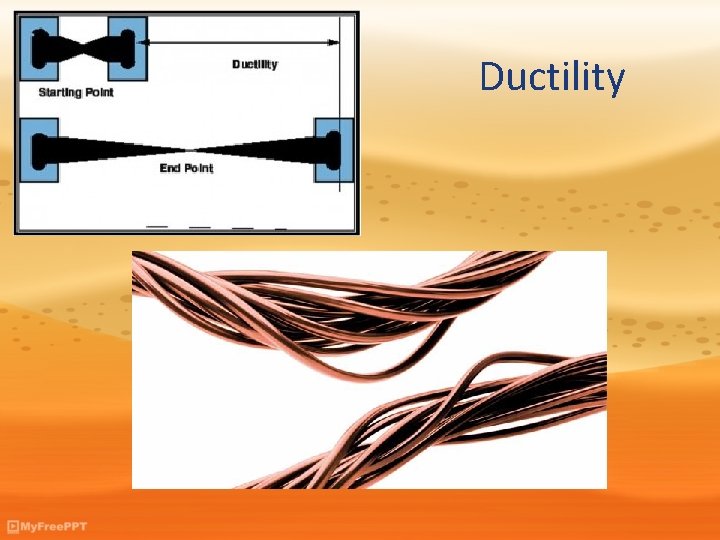
Physical Properties • Thermal Conductivity: The rate at which a substance transfers heat. • State: The physical form in which a substance exists, such as a solid, liquid, gas, plasma. • Solubility: The ability to dissolve in another substance. Ex: Flavored drink dissolves in H 2 O. • Ductility: The ability of a substance to be pulled into a wire. Ex: Copper is often used to make wire because it is ductile. • Malleability: The ability of a substance to be rolled or pounded into thin sheets. Ex: Aluminum foil. • Density: The ration of the mass of a substance to the volume of the substance.

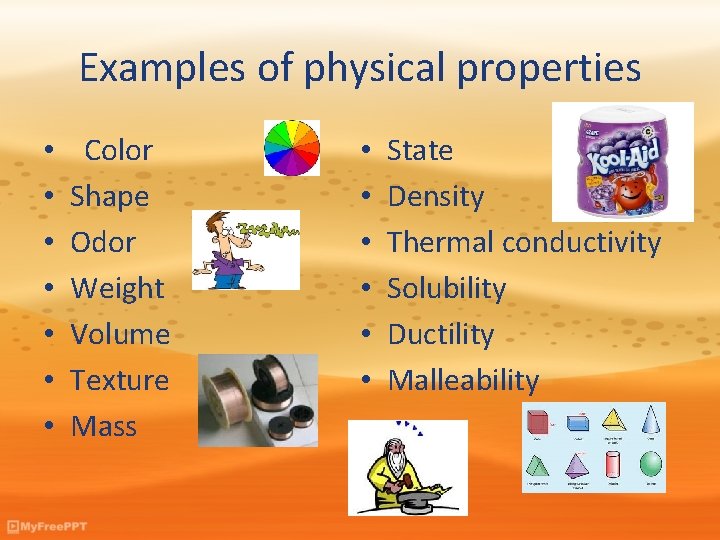
Examples of physical properties • • Color Shape Odor Weight Volume Texture Mass • • • State Density Thermal conductivity Solubility Ductility Malleability

Ductility

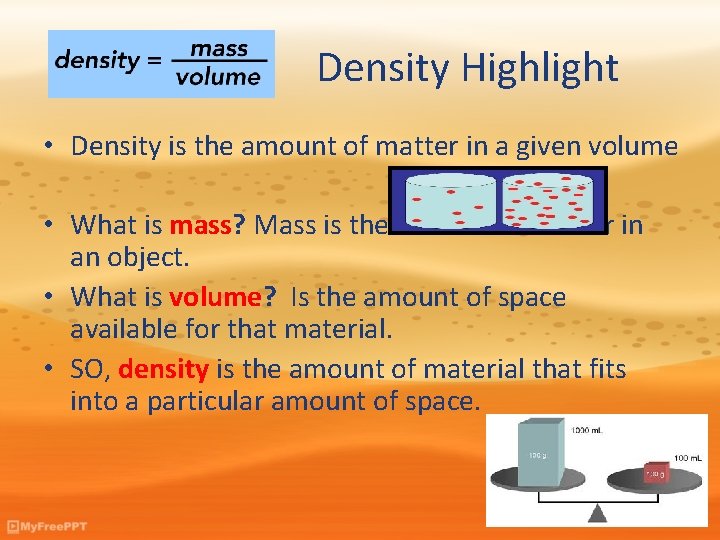
Density Highlight • Density is the amount of matter in a given volume • What is mass? Mass is the amount of matter in an object. • What is volume? Is the amount of space available for that material. • SO, density is the amount of material that fits into a particular amount of space.

Density Continued • Different materials have different densities. • EVERY bit of material/substance has a specific density that is the same no matter how much of it you have or from where you get the sample.

Sink or Float • Density determines whether a substance will sink or float. • More dense= will sink • Less dense= will float.

Chemical properties • A Chemical property is a characteristic of a substance that can only be observed by changing it into a different substance.

Chemical Properties Describes matter based on its ability to change into new matter that has different properties. • Examples of Chemical Properties: Flammability: The ability of a substance to burn. Reactivity: The ability of two or more substances to combine and form one or more new substances.

Chemical properties - Examples – The ability to burn – Ability to tarnish – Ability to rust – Ability to decompose – Ability to react with other chemicals – Instability – Ability to do acid/base reactions

Examples 1. Flammability 2. Reactivity 3. Combustibility: The measure of how easily a substance will set on fire, through fire or combustion.

• Titanium is very strong and doesn’t rust, so it is often used in jet engines. • Titanium is also nonallergenic. This, combined with the fact that it is rust proof makes it great for artificial joints as well as piercings. Chemical and physical properties – So what?

Chemical and physical properties – So what? • Helium is almost completely nonreactive (inert). • It is lighter than air, so it’s great for floating balloons (or making funny voices. ) • When electricity runs through helium, it glows a creamy pale peach color.

Chemical and physical properties – So what? • In 1943, all US pennies were made of zinc plated steel because copper was being used in the war. The pennies had to be coated with zinc because steel will rust, but zinc won’t.

Chemical and physical properties – So what? • Sulfur smells awful. Rotten eggs, onions, and garlic all have sulfur in them. Stink bombs use sulfur to create a bad smell. • Sulfur is also flammable, and it is one of the 3 main ingredients in gun powder.

Chemical and physical properties – So what? • Chromium is famous for its intense luster. Chrome plated tools, jewelry, silverware, or car parts are very popular.

Law of Conservation of Matter • Matter cannot be destroyed nor created • Originally called the Law of Conservation of Mass. • Antoine Laurent Lavoisier is given credit for this discovery. • Einstein stated that the Law of Conservation of Matter was the same as the Law of Conservation of energy. Energy is in matter and matter is energy. E=mc 2…(energy x mass x speed of light squared).

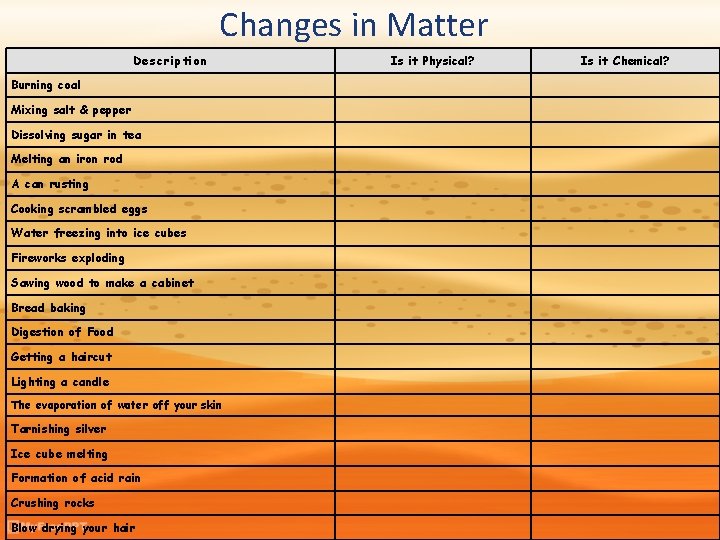
Changes in Matter • All matter, regardless of state, undergoes physical and chemical changes. – Physical changes-will change the visible appearance without changing the composition of the matter. – Chemical changes-will change the composition of matter and will create a new substance. These changes are irreversible.

Changes in Matter Description Burning coal Mixing salt & pepper Dissolving sugar in tea Melting an iron rod A can rusting Cooking scrambled eggs Water freezing into ice cubes Fireworks exploding Sawing wood to make a cabinet Bread baking Digestion of Food Getting a haircut Lighting a candle The evaporation of water off your skin Tarnishing silver Ice cube melting Formation of acid rain Crushing rocks Blow drying your hair Is it Physical? Is it Chemical?
 Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Now all has been heard here is the conclusion of the matter
Now all has been heard here is the conclusion of the matter Anything that has mass and volume
Anything that has mass and volume Anything that takes up space
Anything that takes up space Name 3 points
Name 3 points Intensive and extensive properties
Intensive and extensive properties Chemical property of matter
Chemical property of matter Properties of liquid state
Properties of liquid state Properties of matter vocabulary
Properties of matter vocabulary Classification of matter concept map
Classification of matter concept map Objectives of properties of matter
Objectives of properties of matter General properties of matter
General properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Example of measurable properties
Example of measurable properties States of matter jeopardy
States of matter jeopardy Matter jeopardy
Matter jeopardy Matter definition
Matter definition Matter and its properties
Matter and its properties Classification graphic organizer examples
Classification graphic organizer examples The study of composition structure and properties of matter
The study of composition structure and properties of matter Chemical properties of matter
Chemical properties of matter Properties of matter section 2
Properties of matter section 2 Matter-properties and changes answer key
Matter-properties and changes answer key Properties of matter vocabulary
Properties of matter vocabulary