Matter their SI units All matter has two









- Slides: 12

Matter & their SI units • All matter has two properties. • All matter has mass • • • All matter takes up space (has volume) • • The SI unit for mass is the kilogram (Kg) One Kg equals about 2. 2 lbs The SI unit for volume is the liter (L) One liter is a cubic decimeter. This rule will be bent later when talking about electrons (e-) Other SI units include sec, J, Hz.

Making Measurements • One must use units when making any measurement. • All measurements include one uncertain digit. – An uncertain digit is a guess and can vary from one person to another. – The uncertain digit is the last number recorded. It is a number that makes the measurement one number more accurate than the measuring device itself.

Making Measurements • All measurements have two kinds of numbers. – Those that are considered place holders & – Those that are considered significant

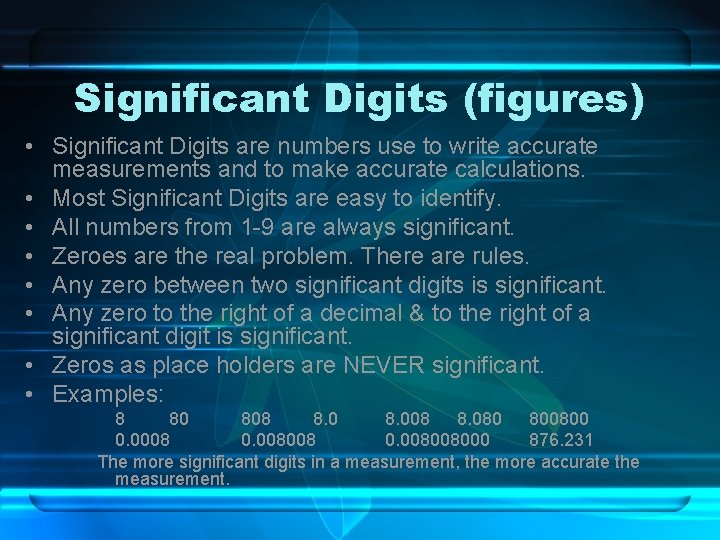
Significant Digits (figures) • Significant Digits are numbers use to write accurate measurements and to make accurate calculations. • Most Significant Digits are easy to identify. • All numbers from 1 -9 are always significant. • Zeroes are the real problem. There are rules. • Any zero between two significant digits is significant. • Any zero to the right of a decimal & to the right of a significant digit is significant. • Zeros as place holders are NEVER significant. • Examples: 8 80 808 8. 008 8. 080 800800 0. 0008 0. 008008000 876. 231 The more significant digits in a measurement, the more accurate the measurement.

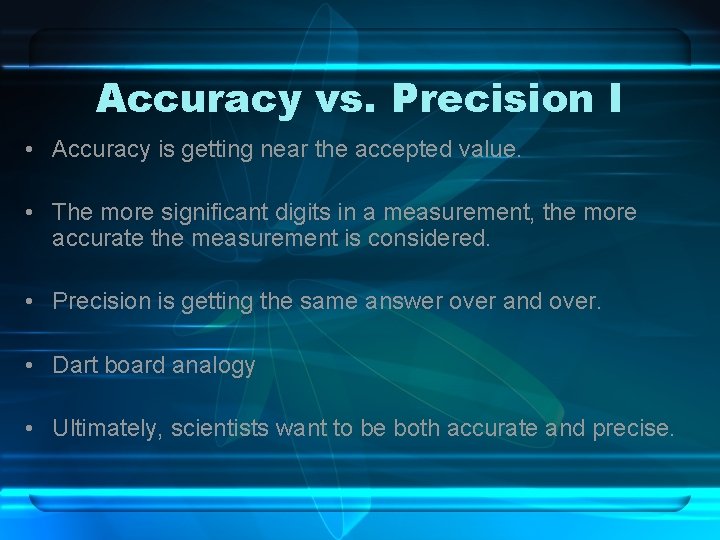
Accuracy vs. Precision I • Accuracy is getting near the accepted value. • The more significant digits in a measurement, the more accurate the measurement is considered. • Precision is getting the same answer over and over. • Dart board analogy • Ultimately, scientists want to be both accurate and precise.

Accuracy vs. Precision II • When calculations are made, an answer can never be more accurate (meaning having more sig. figs) than the least accurate number. • Answers must be written to reflect this rule. • 203. 66 cm x 43 cm = 8757. 38 cm 2 • This answer must contain only 2 digits.

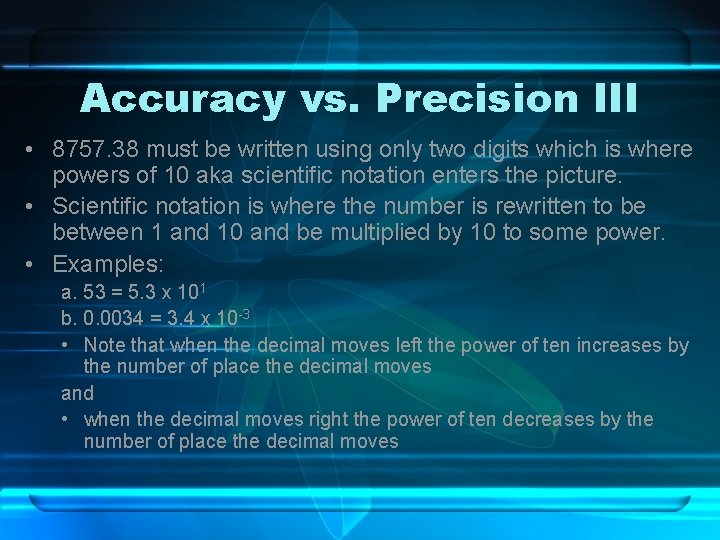
Accuracy vs. Precision III • 8757. 38 must be written using only two digits which is where powers of 10 aka scientific notation enters the picture. • Scientific notation is where the number is rewritten to be between 1 and 10 and be multiplied by 10 to some power. • Examples: a. 53 = 5. 3 x 101 b. 0. 0034 = 3. 4 x 10 -3 • Note that when the decimal moves left the power of ten increases by the number of place the decimal moves and • when the decimal moves right the power of ten decreases by the number of place the decimal moves

Powers of Ten Practice • 5. 6 x 100 =. 0032 = 5. 6 3. 2 x 10 -3 • 5. 6 x 103 = 5. 6 x 10 -2 = 5600 0. 056 • 8. 00 x 102 = 10 -2 = 800. 0. 01 • 76. 43 = 7. 643 x 101 or 7. 643 x 10 • So then 8757. 38 cm 2 would be written 8. 8 x 103 cm 2 • If the 43 were more accurate we would have more significant digits in the answer.

• How can I write 8757. 38 cm 2 to have only 2 digits – 1 – 2

Rounding 5’s I • Finally the last math in chemistry item. • This is one where chemistry and math do not agree. • What do we do when we have to round 5 ’s? • Math rounds 5’s up. They have a zero. • If you recall in slide #1, all matter must have mass and volume, therefore there is no such number as zero. Chemistry rounds 5’s to make the uncertain digit even. • Why?

Rounding 5’s II • If we have no zero in chemistry, then • 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 0 never really starts or stops. • If you round 5’s up you are always having error on the high side. Since there are just as many numbers below 5 as above 5 this is not very accurate.

Rounding 5’s III • Proof • Here is the problem: We want to round to nearest tenth. • 18. 65 + 19. 75 = • Calculator answer • 18. 65 + 19. 75 = 38. 40 = 38. 4 • Math way: Ø 18. 7 + 19. 8 = 38. 5 • Chemistry way: Ø 18. 6 + 19. 8 = 38. 4