MATTER Properties of Matter Two classes of properties























- Slides: 31

MATTER

Properties of Matter • Two classes of properties: • Physical • Chemical • Physical: observable without changing composition of substance • Chemical: only observed if a change in composition occurs

Properties of Matter cont… • Two types of physical properties: • Extensive • Intensive • Extensive: properties that depend on the amount of material is present • Intensive: depends on the identity of the substance only, not the amount present


Physical Changes • A change of matter from one form to another without changing the substance itself. • Examples: phase changes, mixtures • Heating Curves: Lab

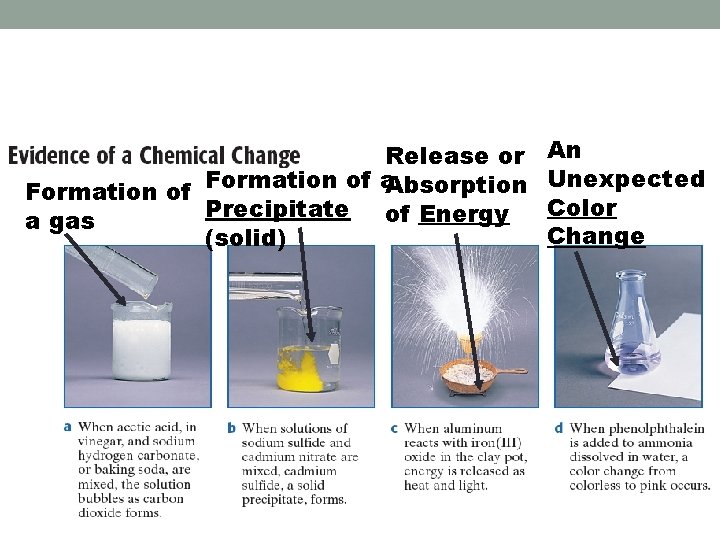
Chemical Changes • Entirely new substances with different properties • A + B C (reactants go to products)

Release or An Unexpected Formation of a Absorption Formation of Color Precipitate of Energy a gas Change (solid)

Chemical or Physical • Frying an egg • Chemical • Boiling Water • Physical • Sanding a wooden • Physical plank • Digesting food • Popping a balloon • Chemical • Physical

Properties and Changes Practice • Properties: identify each as • Changes: identify each as chemical or physical 1. Silver tarnishes 2. Copper can be pounded into a bowl 3. Helium is unreactive 4. Barium melts at 725°C 5. Potassium metal reacts violently with water chemical or physical 1. Water condensates 2. Electricity changes water into hydrogen and oxygen 3. Yeast cells in bread make carbon dioxide and ethanol from sugar 4. Wood burns 5. Copper wire turns green over time

ATOM • Atom – the smallest unit of an element that maintains the properties of that element.

Pure Substance • A sample of matter, either a single element or a single compound, that has definite chemical and physical properties Figure 14, Page 22

Elements • A pure substance • All atoms of the same element have the same atomic number

Compounds • A pure substance • Two or more different elements joined by chemical bonds.

Molecules • The smallest unit of a substance • Has physical and chemical properties of that substance.

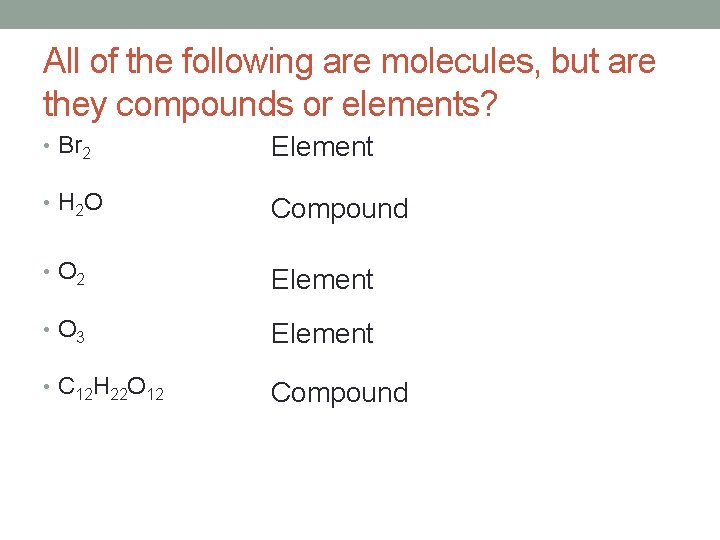
All of the following are molecules, but are they compounds or elements? • Br 2 Element • H 2 O Compound • O 2 Element • O 3 Element • C 12 H 22 O 12 Compound

Mixtures • A combination of two or more substances • Not chemically combined • Examples are air, ice tea, and even cake batter • Proportions can vary

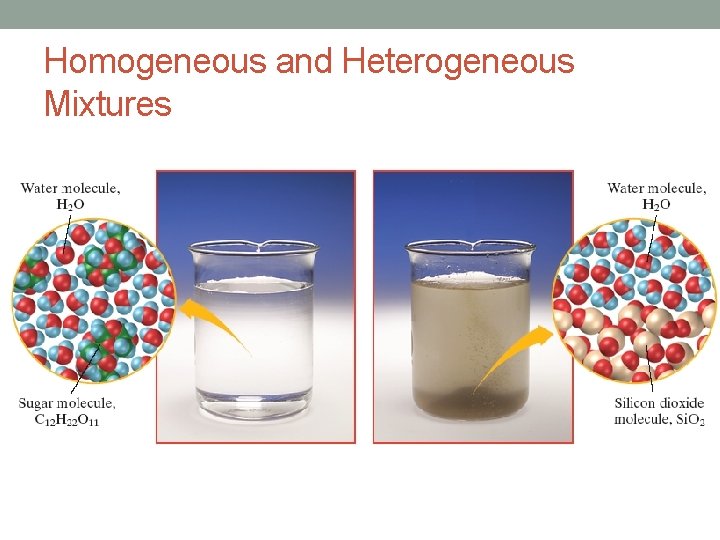
Homogeneous Mixtures • Uniform distribution • Same proportions of ingredients • Examples: Gasoline, air, and syrup

Heterogeneous Mixtures • Not uniformly distributed. • Different proportions. • Examples: Chocolate chip cookie dough, vegetable soup and granite.

Homogeneous and Heterogeneous Mixtures


Classification Practice Classify the following as either a pure substance or a mixture. If a pure substance, is it an element or a compound? If a mixture, homogeneous or heterogeneous? 1. Concrete 6. Hamburger 2. Sucrose (table sugar) 7. Copper 3. Diamond 8. Copper (II) oxide 4. Saltwater 9. Milk 5. Dry ice (solid CO 2) 10. Vitamin C

STATES OF MATTER

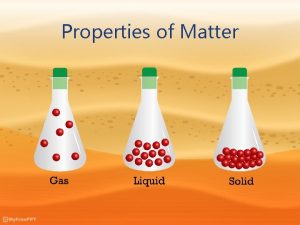
There are Four States of Matter • Solid • Liquid • Gas

Solids • Particles are very close together • Have orderly, fixed arrangements • Fixed volumes • Particles can only vibrate in position

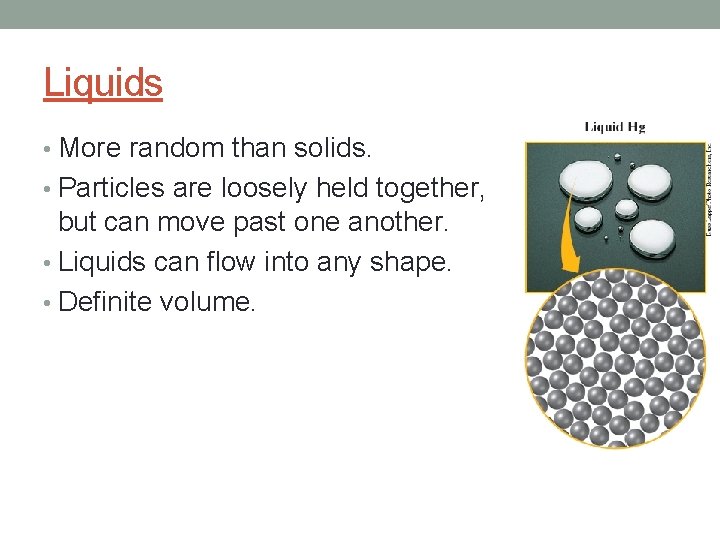
Liquids • More random than solids. • Particles are loosely held together, but can move past one another. • Liquids can flow into any shape. • Definite volume.

Attractive forces between liquid particles may result in: • Cohesion • Attraction for each other • Adhesion • Attraction to other materials • Capillary Action • Ability to “climb” due to cohesion and adhesion • Surface Tension • Force that act on the surface of a liquid and that tends to minimize the area of the surface

Gas • Essentially independent particles. • Large space between particles little to no attraction between particles. • Gases can flow into any shape • No definite volume

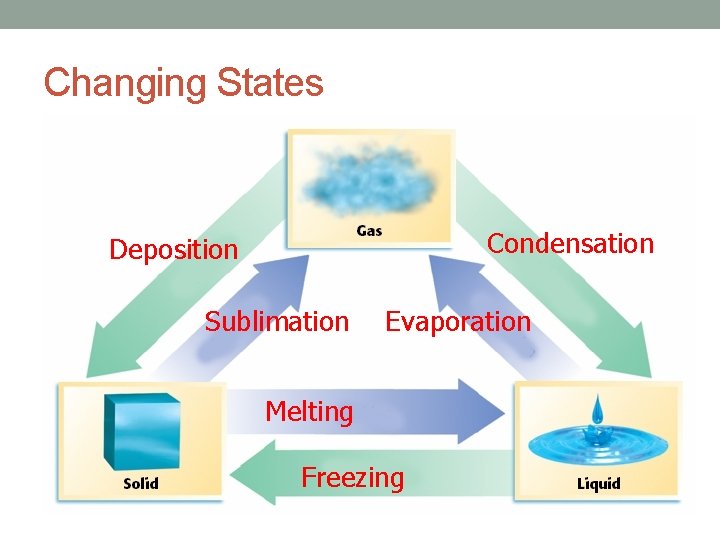
Changing States Condensation Deposition Sublimation Evaporation Melting Freezing

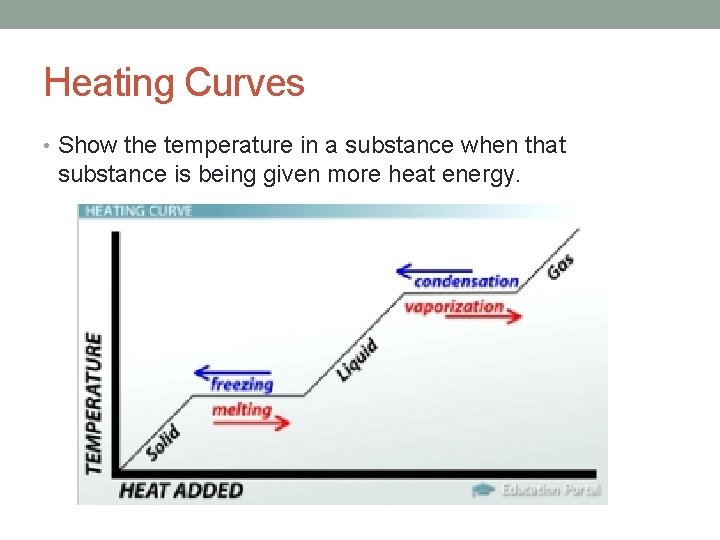
Heating Curves • Show the temperature in a substance when that substance is being given more heat energy.

SEPARATING MIXTURES Since mixtures are just physically combined, they can be separated.

Separating Mixtures • Filtering – separation through differences in particle size • Decanting –separating by pouring • Distillation –separate two liquids based on differences in boiling points • Evaporation – removing a liquid to leave a solid • Chromatography – Separates by using a mobile phase and a stationary phase
 Subclasse das palavras
Subclasse das palavras Pre ap classes vs regular classes
Pre ap classes vs regular classes Name two categories used to classify properties of matter
Name two categories used to classify properties of matter 3 classes of matter
3 classes of matter People matter survey
People matter survey What are the three classes of yeast breads
What are the three classes of yeast breads Weber social class
Weber social class Direct link network
Direct link network A proposition that relates two classes
A proposition that relates two classes Section 1 composition of matter
Section 1 composition of matter Meysam golmohammadi
Meysam golmohammadi Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Optic tract
Optic tract Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter Matter
Matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Do
Do Properties of matter vocabulary
Properties of matter vocabulary Matter concept map
Matter concept map Objectives of properties of matter
Objectives of properties of matter General property of matter
General property of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Examples of chemical properties of matter
Examples of chemical properties of matter Properties of matter jeopardy
Properties of matter jeopardy Matter jeopardy
Matter jeopardy Matter definition
Matter definition Matter and its properties
Matter and its properties Classification of matter graphic organizer
Classification of matter graphic organizer Study of the composition structure and properties
Study of the composition structure and properties