Properties Of Matter Classifying Matter Phases of Matter

































































- Slides: 69



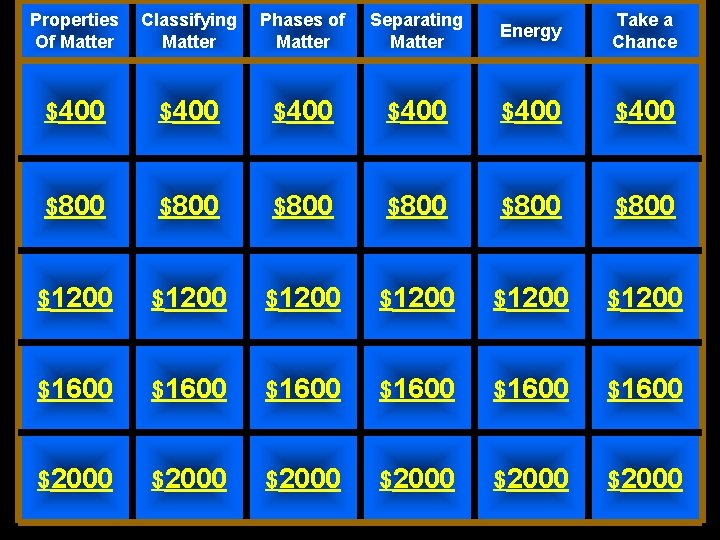
Properties Of Matter Classifying Matter Phases of Matter Separating Matter Energy Take a Chance $400 $400 $800 $800 $1200 $1200 $1600 $1600 $2000 $2000

Final Jeopardy Particle Diagrams

Final Jeopardy Draw the product of an endothermic physical change

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$200 answer question

$400 answer question

$600 answer question

$800 answer question

$1000 answer question

$400 3. melting point of 1930 C Which of the following would be considered a physical property? 1. reacts with an acid 2. flammable 3. melting point of 1930 C 4. corrodes easily

$800 4. flammable Which of the following would be considered a chemical property 1. malleable 2. boils at 1000 C 3. low melting point 4. flammable

$1200 physical Is dissolving Na. Cl in water a physical or chemical change?

$1600 Chemical = identity Physical = appearance (look) A chemical change occurs when the _______ of a substance changes and a physical change occurs when the _____ of a substance changes.

$2000 Formation of a gas (bubble), color change, and formation of a percipitate Two evidences that a chemical reaction has occurred are the presence of heat and light. What are the other 3?

$400 Pure substance Matter can be classified as a pure substance or a mixture. If it can NOT be physical separated, it is a ______

$800 3. Salt and sand Which of the following is a mixture? 1. salt (Na. Cl) 2. gold (Au) 3. salt and sand (Na. Cl + Si. O 2) 4. helium gas (He (g))

$1200 Elements: He, N 2, Ag Compounds: Na. Cl Element or Compound 2. Place the following under the correct column salt (Na. Cl) Nitrogen (N 2) silver (Ag) helium gas (He (g))

$1600 air Which of the following is a mixture? 1. salt (Na. Cl) 2. sugar (C 12 H 22 O 11) 3. lead (Pb) 4. air

$2000 4 The following particle diagrams represents 1. Mixture of elements only 2. Pure substance 3. Mixture of compounds only 4. Mixture of compounds and elements

$400 Has mass and takes up space Define Matter

$800 gas Name the phase of matter shown below

$1200 3 Solids have 1. definite shape and indefinite volume 2. indefinite shape and indefinite volume 3. definite shape and definite volume 4. indefinite shape and definite volume

$1600 Definite volume and indefinite shape Use the word(s) indefinite and/or definite Liquids have ____ volume _____ shape

$2000 1. Physical change 2. freezing 2 parts 1. When a liquid becomes a solid, what type of change has taken place (physical or chemical)? 2. What is this phase change called?

$400 sorting Peas and carrots can be separated by this technique

$800 Filtration This mixture can be separated using which technique?

$1200 3. Salad dressing An example of a heterogeneous mixture is: 1. water 2. vegetable oil 3. salad dressing 4. vinegar

$1600 physical Mixture can be separated based on _____ properties

$2000 homogeneous When salt it dissolved in water, the individual salt particles are too small to see, so this type of mixture is called a _______ mixture

$400 energy _____ is the ability to do work

$800 J (joule) The SI unit of energy is _______

$1200 Exothermic Endothermic or Exothermic When energy exits the system and warms the environment, it’s considered to be _______

$1600 endothermic Endothermic or Exothermic When energy enters the system it (and it becomes warm) and the environment cools, it’s considered to be _______

$2000 1. Endo 2. Exo 3. Exo Name the following as endothermic or exothermic 1. Solid liquid 2. Gas liquid 3. Liquid solid

$400 compound A substance that consists of only 2 or more type of elements is called a _______

$800 element A substance that consists of only one type of atom is called a(n) ______

$1200 PE State whether the following describe potential energy (PE) or kinetic energy (KE) a carrot ____________

$1600 PE State whether the following describe potential energy (PE) or kinetic energy (KE) a dam holding back a river of water ____________

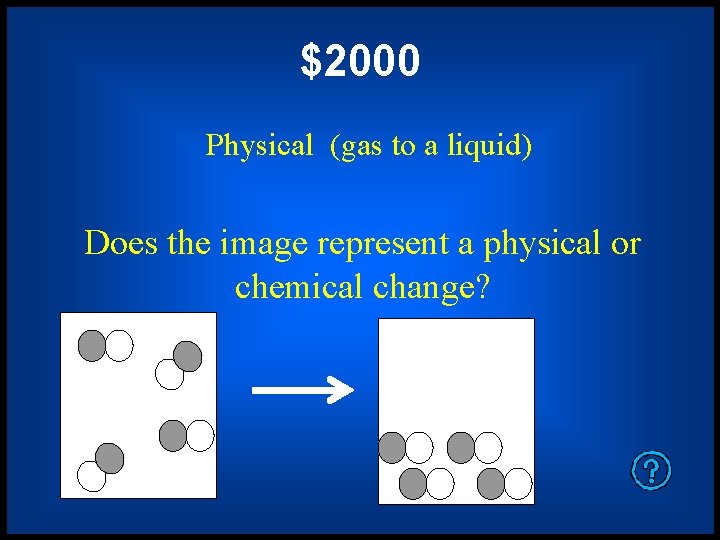
$2000 Physical (gas to a liquid) Does the image represent a physical or chemical change?

Daily Double answer question

Daily Double answer question

Daily Double answer question

The Jeopardy champion!
 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Classification of matter flowchart
Classification of matter flowchart What can matter be classified as
What can matter be classified as Classification of matter quiz
Classification of matter quiz Classifying matter quiz
Classifying matter quiz What is classification of matter
What is classification of matter Physical change concept map
Physical change concept map Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer for matter
Graphic organizer for matter Worksheet classification of matter
Worksheet classification of matter Phases of matter foldable
Phases of matter foldable Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases 4 phases of matter
4 phases of matter Phases of matter
Phases of matter Phases changes of matter
Phases changes of matter Phase change concept map
Phase change concept map 3 phases of matter
3 phases of matter Four states of matter
Four states of matter Physical properties of ice cube
Physical properties of ice cube Extensive and intensive examples
Extensive and intensive examples Electrical properties of matter
Electrical properties of matter Physical properties of notebook paper
Physical properties of notebook paper Physical properties of matter jeopardy
Physical properties of matter jeopardy Properties of matter vocabulary
Properties of matter vocabulary Measurable properties of matter
Measurable properties of matter Kwl chart properties of matter
Kwl chart properties of matter Properties of matter elasticity
Properties of matter elasticity Big idea 8 properties of matter
Big idea 8 properties of matter 2 properties of matter
2 properties of matter General property of matter
General property of matter Properties of matter objectives
Properties of matter objectives Useful but harmful materials
Useful but harmful materials General properties of solids
General properties of solids What is matter in science grade 7
What is matter in science grade 7 Matter and its properties
Matter and its properties Properties of matter jeopardy
Properties of matter jeopardy How do you describe matter
How do you describe matter Properties of liquid matter
Properties of liquid matter Matter in natural science
Matter in natural science Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Classification and properties of matter
Classification and properties of matter Conductivity
Conductivity Chapter 2 properties of matter answer key
Chapter 2 properties of matter answer key Properties and characteristics of matter
Properties and characteristics of matter Study of the composition structure and properties
Study of the composition structure and properties Properties of matter jeopardy
Properties of matter jeopardy Intrinsic properties of matter
Intrinsic properties of matter Properties of matter vocabulary
Properties of matter vocabulary Is brittleness a physical or chemical property
Is brittleness a physical or chemical property Properties of matterwhat is matter?
Properties of matterwhat is matter? Properties of water in matter
Properties of water in matter Matter-properties and changes answer key
Matter-properties and changes answer key Properties and changes of matter worksheet
Properties and changes of matter worksheet Properties of matter wave
Properties of matter wave Bamboo basket properties of matter
Bamboo basket properties of matter General property of matter
General property of matter Volume properties of matter
Volume properties of matter What are chemical properties of matter
What are chemical properties of matter Objectives of properties of matter
Objectives of properties of matter Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray and white matter
Gray and white matter Gray matter and white matter
Gray matter and white matter Composition of matter section 1
Composition of matter section 1 Matter
Matter What makes up the diencephalon
What makes up the diencephalon Write all classifications that apply to the real number 4
Write all classifications that apply to the real number 4 Classifying triangles in the coordinate plane
Classifying triangles in the coordinate plane