Matter Properties Change A Matter MATTER ANYTHING THAT


























- Slides: 51

Matter: Properties & Change

A. Matter • MATTER – ANYTHING THAT HAS MASS AND TAKES UP SPACE – EVERYTHING AROUND US • CHEMISTRY – THE STUDY OF MATTER AND THE CHANGES IT UNDERGOES

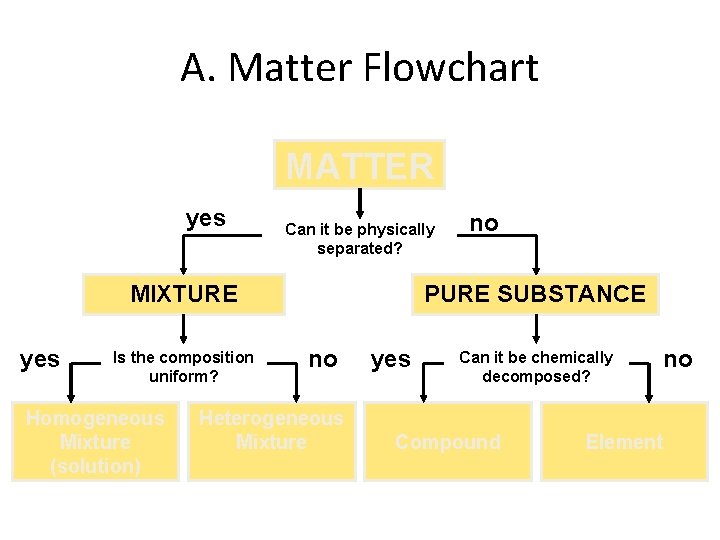
A. Matter Flowchart MATTER yes Can it be physically separated? MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element

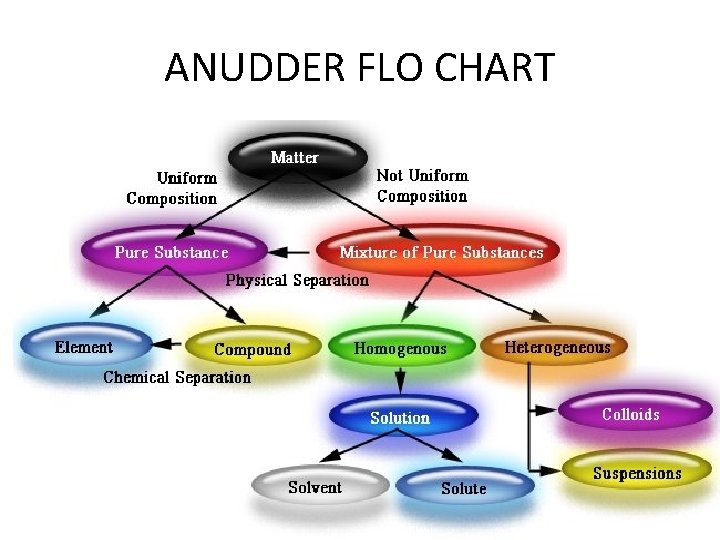
ANUDDER FLO CHART

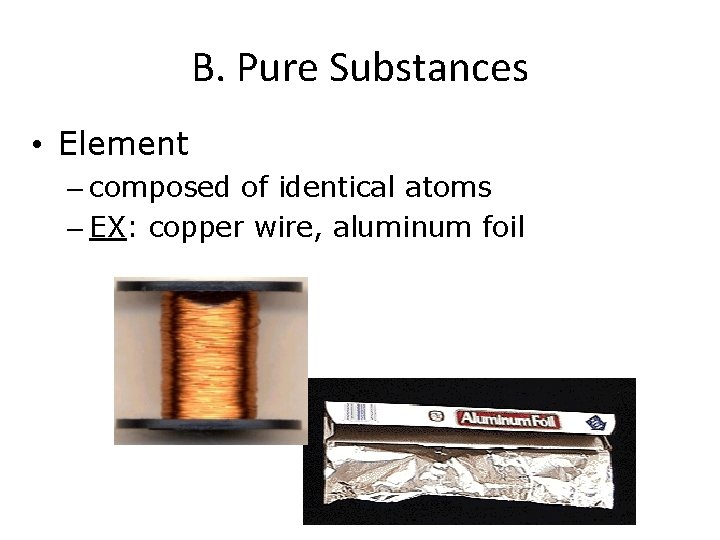
B. Pure Substances • Element – composed of identical atoms – EX: copper wire, aluminum foil

B. Pure Substances • Compound – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl)

C. Mixtures • Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

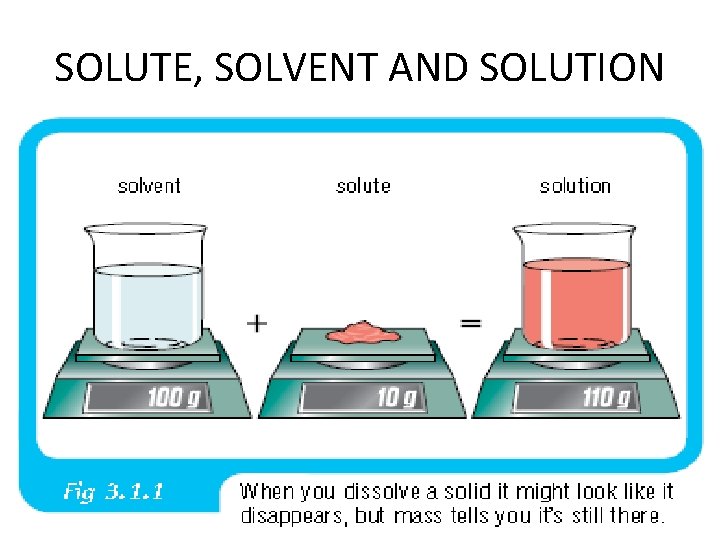
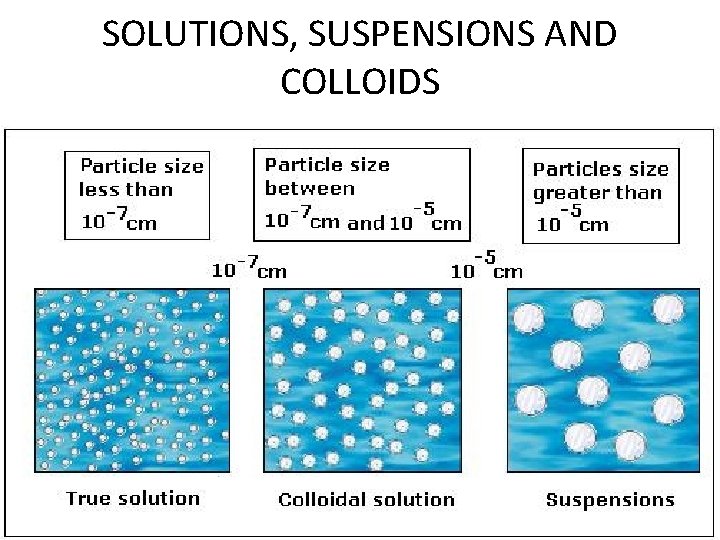
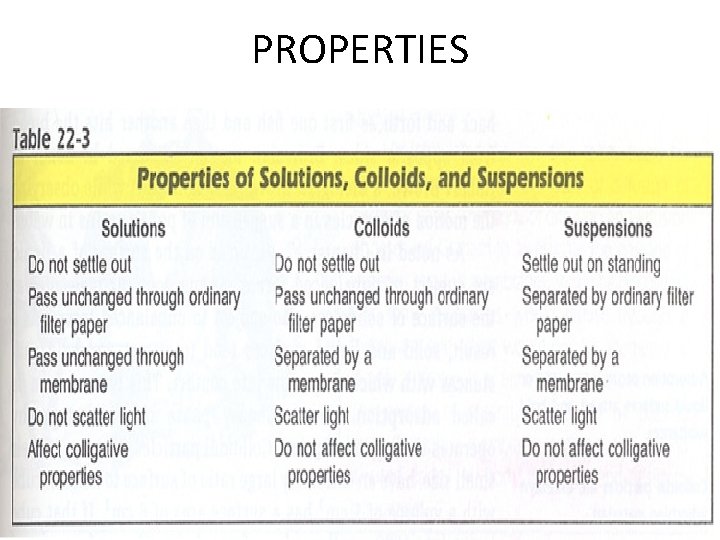
C. Mixtures • Solution – homogeneous – very small particles – particles don’t settle – EX: rubbing alcohol

SOLUTE, SOLVENT AND SOLUTION

DISSOLVING SALT IN WATER

C. Mixtures • Heterogeneous – medium-sized to large-sized particles – particles may or may not settle – EX: milk, freshsqueezed lemonade

SOLUTIONS, SUSPENSIONS AND COLLOIDS

PROPERTIES

C. Mixtures • Examples: • Answers: – tea – Solution – muddy water – Heterogeneous – fog – Heterogeneous – saltwater – Solution – Italian salad dressing – Heterogeneous

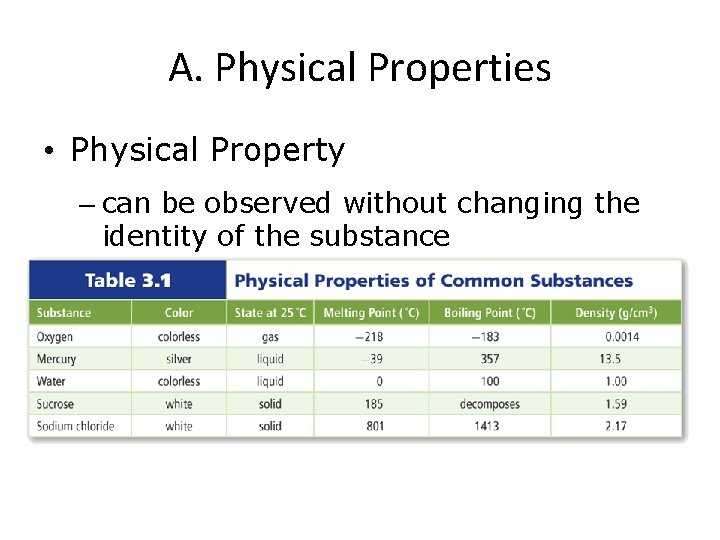
A. Physical Properties • Physical properties can be described as one of 2 types: • Extensive Property – depends on the amount of matter present (example: length) • Intensive Property – depends on the identity of substance, not the amount (example: scent)

A. Physical Properties • Physical Property – can be observed without changing the identity of the substance

F. Physical Changes • Physical Change – changes the form of a substance without changing its identity – properties remain the same • Examples: cutting a sheet of paper, breaking a crystal, all phase changes

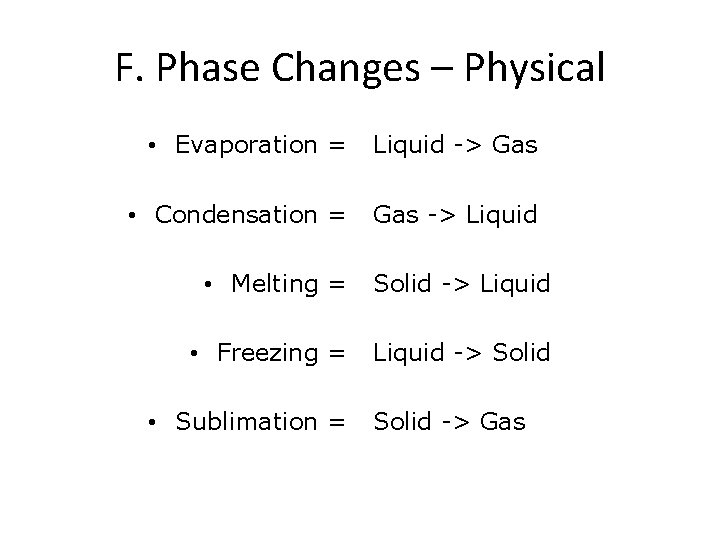
F. Phase Changes – Physical • Evaporation = Liquid -> Gas • Condensation = Gas -> Liquid • Melting = Solid -> Liquid • Freezing = Liquid -> Solid • Sublimation = Solid -> Gas

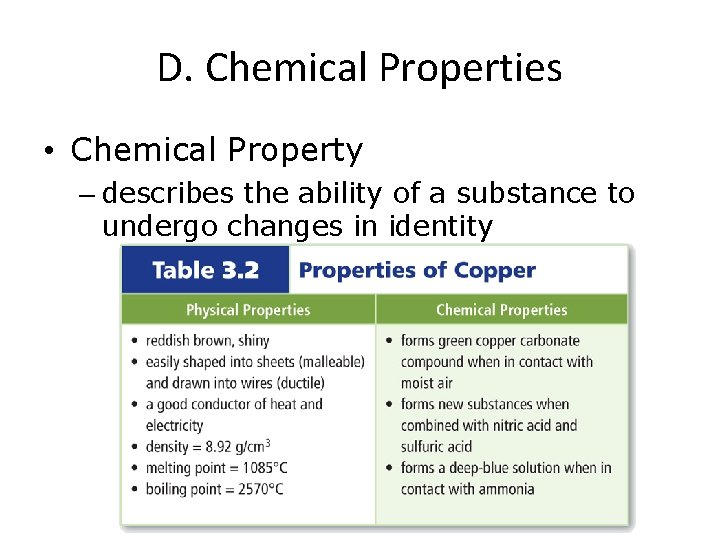
D. Chemical Properties • Chemical Property – describes the ability of a substance to undergo changes in identity

G. Chemical Changes • Process that involves one or more substances changing into a new substance – Commonly referred to as a chemical reaction – New substances have different compositions and properties from original substances

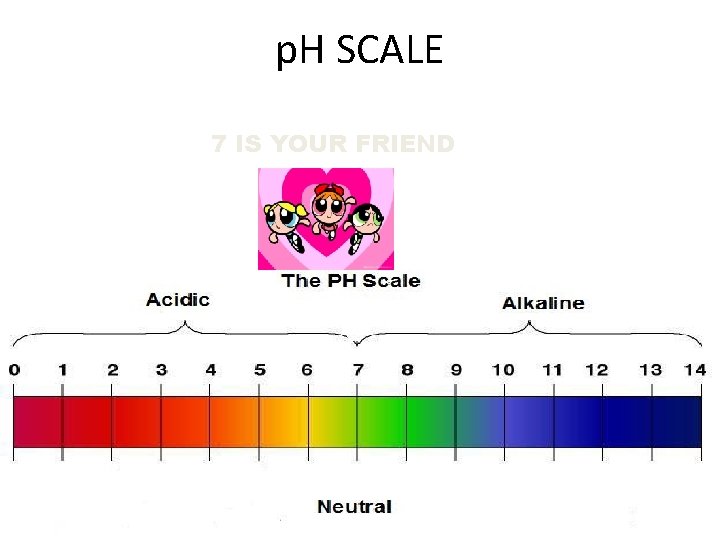
FAMOUS CHEMICAL STUFF U SHOULD KNOW #JIMMY NEUTRON • ACIDS - THINGS THAT RELEASE HYDROGEN IONS ( H + ) WHEN DISSOLVED IN WATER • BASES ( ALKALINE ) - THINGS THAT RELEASE HYDROXIDE IONS ( OH - ) WHEN DISSOLVED IN WATER • THE p. H SCALE MEASURES THE RANGE OF ACIDS/BASES

ACIDS AND BASES

NURD WARNING • PH CAN BE VIEWED AS AN ABBREVIATION FOR POWER OF HYDROGEN - OR MORE COMPLETELY, POWER OF THE CONCENTRATION OF THE HYDROGEN ION. • THE MATHEMATICAL DEFINITION OF PH IS A BIT LESS INTUITIVE BUT IN GENERAL MORE USEFUL. IT SAYS THAT THE PH IS EQUAL TO TO THE NEGATIVE LOGARITHMIC VALUE OF THE HYDROGEN ION (H+) CONCENTRATION, OR • PH = -LOG [H+] • PH CAN ALTERNATIVELY BE DEFINED MATHEMATICALLY AS THE NEGATIVE LOGARITHMIC VALUE OF THE HYDROXONIUM ION (H 3 O+) CONCENTRATION. USING THE BRONSTED-LOWRY APPROACH • PH = -LOG [H 3 O+] • TOLD YA IT WAS NURDY

p. H SCALE 7 IS YOUR FRIEND

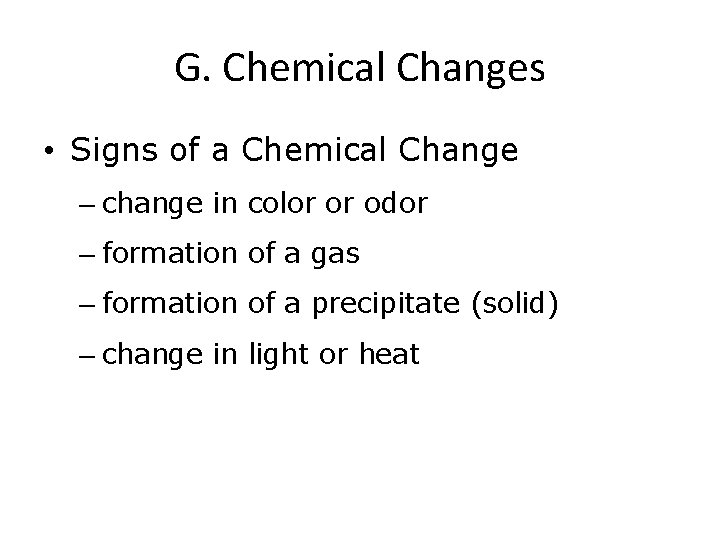
G. Chemical Changes • Signs of a Chemical Change – change in color or odor – formation of a gas – formation of a precipitate (solid) – change in light or heat

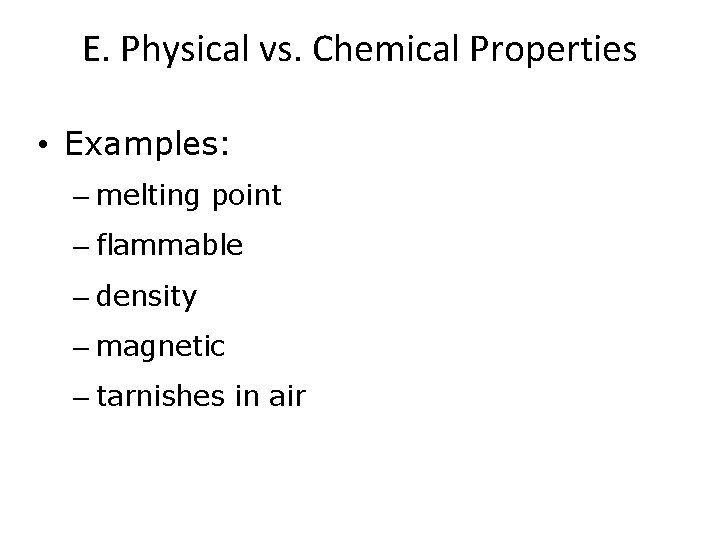
E. Physical vs. Chemical Properties • Examples: – melting point physical – flammable chemical – density physical – magnetic physical – tarnishes in air chemical

H. Physical vs. Chemical Changes • Examples: – rusting iron chemical – dissolving in water physical – burning a log chemical – melting ice physical – grinding spices physical

What Type of Change?

What Type of Change?

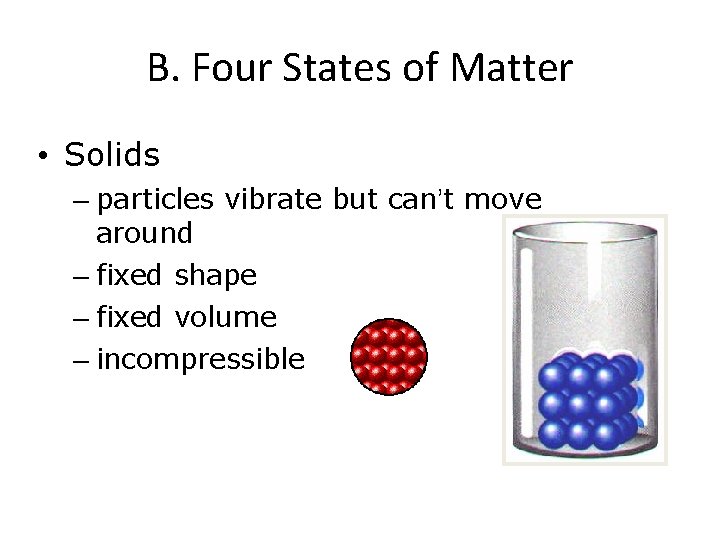
B. Four States of Matter • Solids – particles vibrate but can’t move around – fixed shape – fixed volume – incompressible

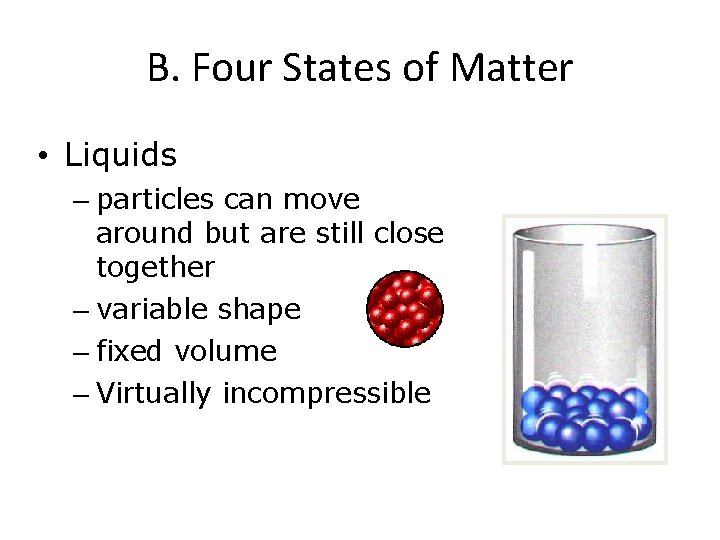
B. Four States of Matter • Liquids – particles can move around but are still close together – variable shape – fixed volume – Virtually incompressible

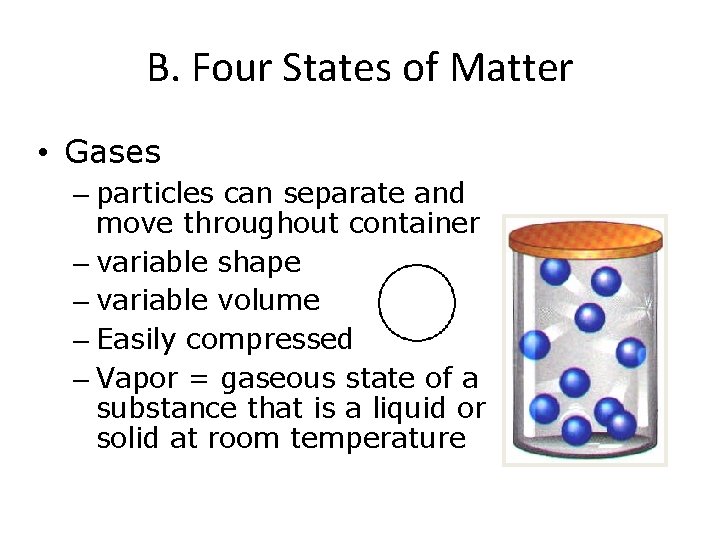
B. Four States of Matter • Gases – particles can separate and move throughout container – variable shape – variable volume – Easily compressed – Vapor = gaseous state of a substance that is a liquid or solid at room temperature

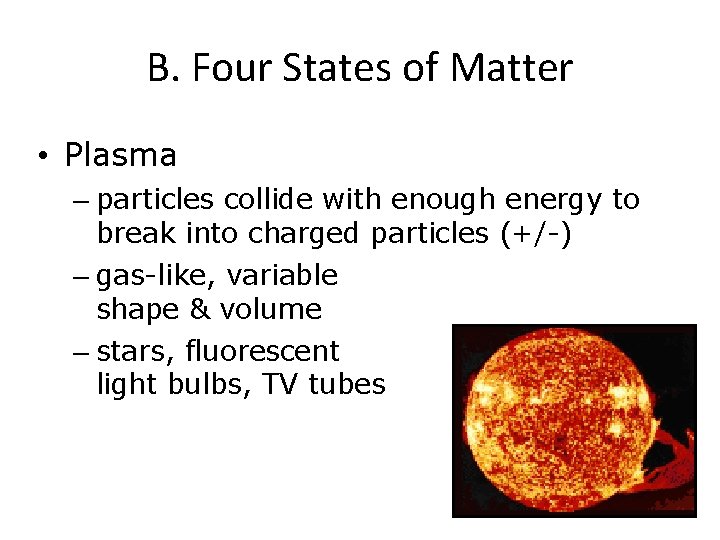
B. Four States of Matter • Plasma – particles collide with enough energy to break into charged particles (+/-) – gas-like, variable shape & volume – stars, fluorescent light bulbs, TV tubes


STATES OF MATTER • The Four States of Matter • Four States • • Solid Liquid Gas Plasma

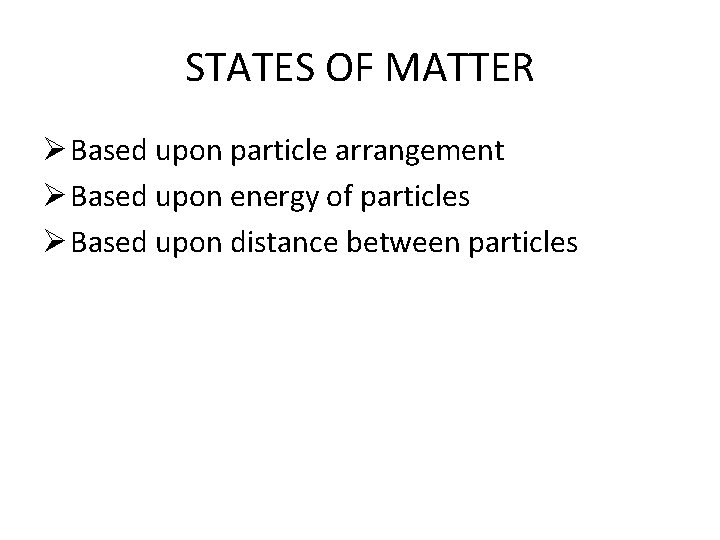
STATES OF MATTER Ø Based upon particle arrangement Ø Based upon energy of particles Ø Based upon distance between particles

Kinetic Theory of Matter is made up of particles which are in continual random motion.

STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume. Heat

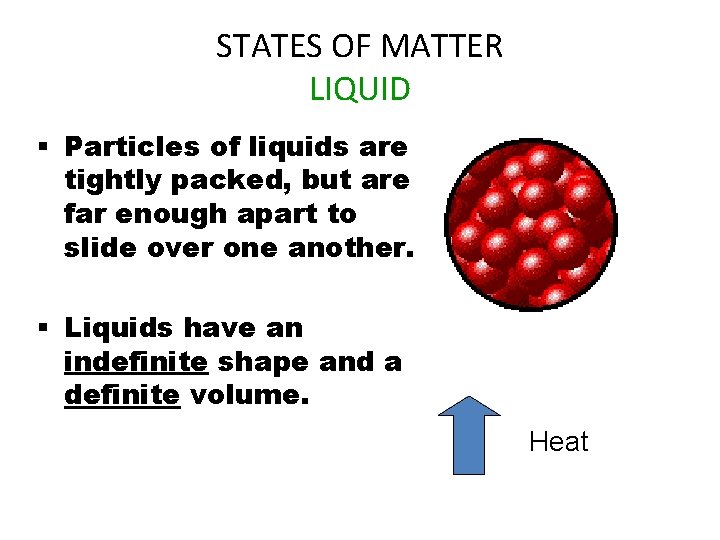
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

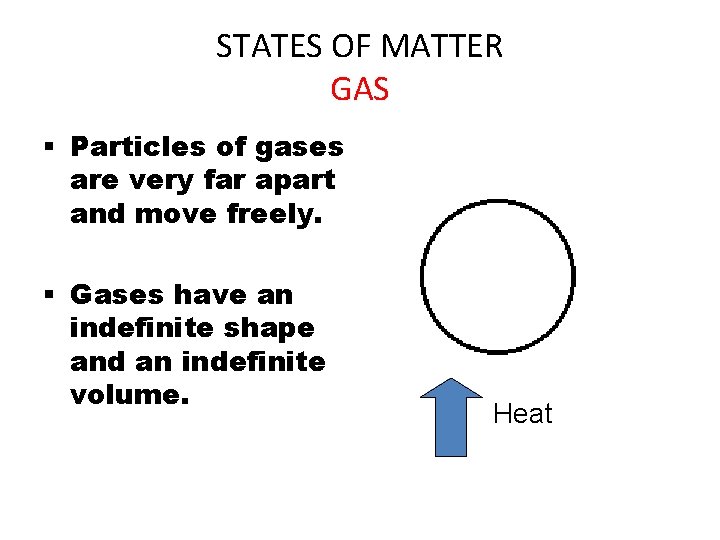
STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

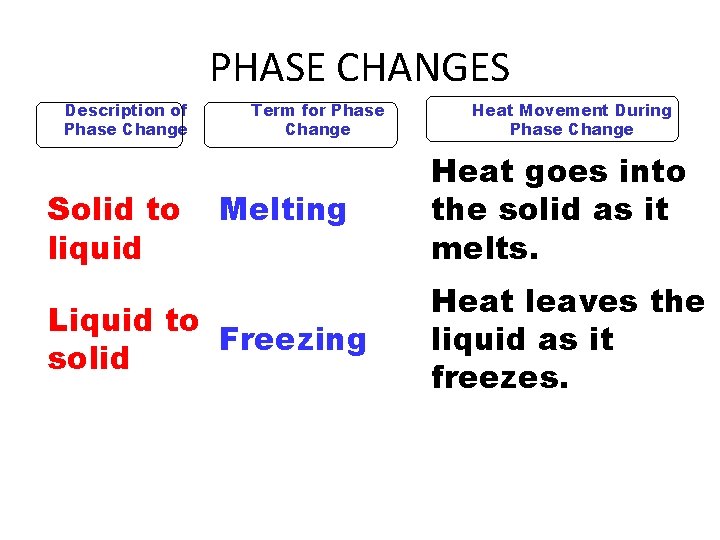
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

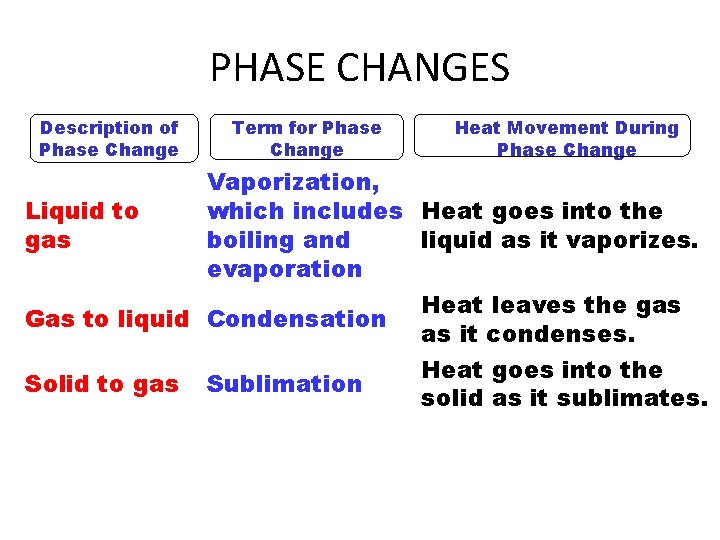
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

LATENT HEAT = HEAT ABSORBED WHEN CHANGING PHASE Specific HEAT CAPACITY = THE AMOUNT OF HEAT NEEDED TO RAISE 1 GRAM OF MATERIAL 1 DEGREE C

But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

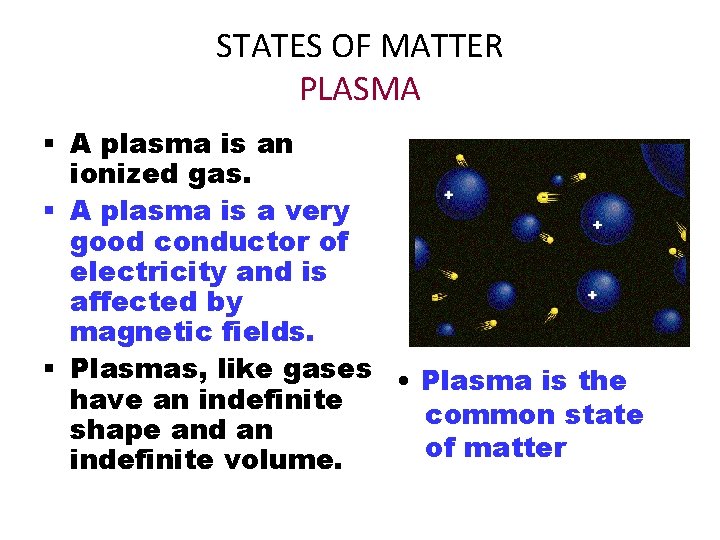
STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

MATTER REVIEW QUIZLET CH 2 QUIZLET CH 3

SAYS TO DO YOUR MATH AND LIKE IT