The Properties of Matter What is matter Matter















- Slides: 23

The Properties of Matter What is matter?

Matter o o o Everything is made of MATTER! Matter is anything that has volume and mass. Volume is the amount of space an object takes up, or occupies.

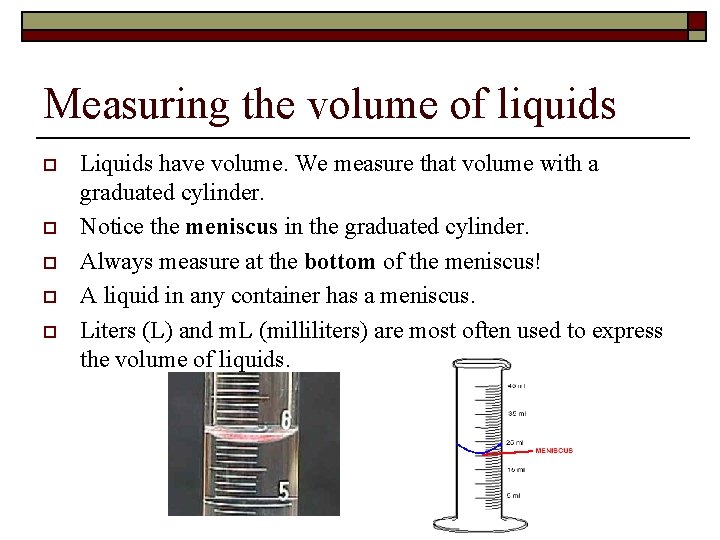
Measuring the volume of liquids o o o Liquids have volume. We measure that volume with a graduated cylinder. Notice the meniscus in the graduated cylinder. Always measure at the bottom of the meniscus! A liquid in any container has a meniscus. Liters (L) and m. L (milliliters) are most often used to express the volume of liquids.

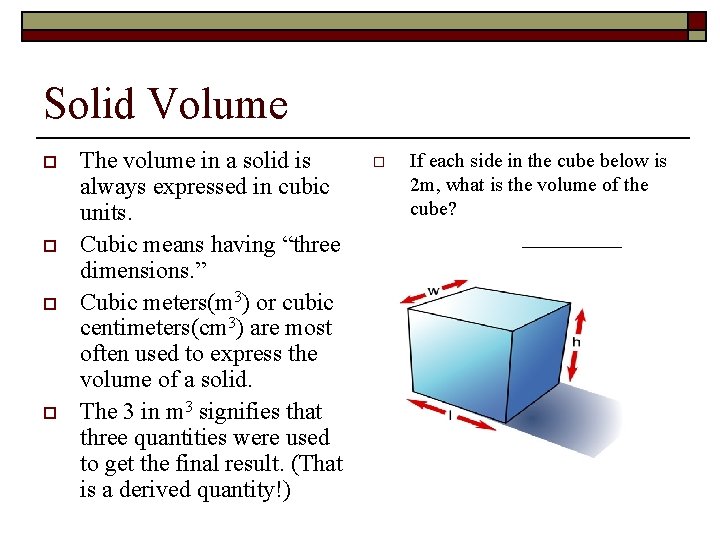
Solid Volume o o The volume in a solid is always expressed in cubic units. Cubic means having “three dimensions. ” Cubic meters(m 3) or cubic centimeters(cm 3) are most often used to express the volume of a solid. The 3 in m 3 signifies that three quantities were used to get the final result. (That is a derived quantity!) o If each side in the cube below is 2 m, what is the volume of the cube? _____

The Volume of Solids, Liquids, and Gases o o 1 m. L = 1 cm 3 REMEMBER THAT! That is why you can compare the volume in liquids to solids. How do you measure the volume of a gas? You can’t see it, so how do you measure it? ex: balloon

Matter and Mass o o o What is mass? Mass is the amount of matter that something is made of. Even atoms have mass! Looking at the picture… The mass stays constant in certain forms of matter such as…_________. The mass changes in certain forms of matter such as…_________.

What is the difference between mass and weight? o o o This is an important concept to understand! Let’s start by understanding gravity. Gravity is the force of attraction between objects that is due to their masses. All matter experiences gravity! The amount of attraction between two objects depends on their weight. There is attraction between all objects with mass, but since they are so small in reference to the earth, the attraction between them is also small.

So, what about weight? o o o Weight is the measure of the gravitational force exerted on an object! Look at Spot and the rock…which one is attracted to the earth more through gravitational force? ________ Which one weighs more? ______ So, this means the greater the gravitational force, the greater the weight. Which weighs more? ------->

Measuring Weight and Mass o o The SI unit for mass is kilogram (kg). Sometimes we will use milligrams or grams. (mg or g) The SI unit for weight (or gravitational force) is NEWTONS. A Newton is approximately equal to the weight of a 100 gram mass on earth.

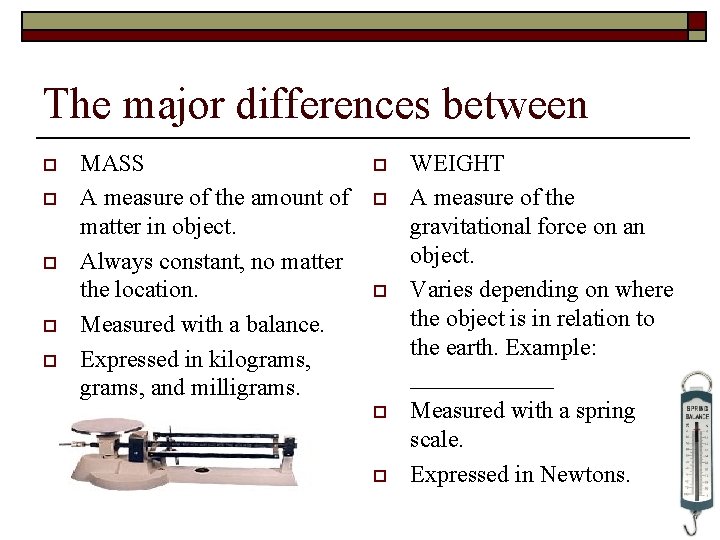
The major differences between o o o MASS A measure of the amount of matter in object. Always constant, no matter the location. Measured with a balance. Expressed in kilograms, and milligrams. o o o WEIGHT A measure of the gravitational force on an object. Varies depending on where the object is in relation to the earth. Example: ______ Measured with a spring scale. Expressed in Newtons.

Describing Matter • • Knowing the characteristics or properties of an object can help you identify the object. There are: • • Physical Properties Chemical Properties

Physical Properties o o o Things that describe the object are physical properties. Physical properties can also be observed or measured without changing the identity of the matter. Examples of physical properties include: color, odor, size, state, density, solubility, melting point, etc…

Chemical Properties o o o Chemical properties describe a substance based on its ability to change into a new substance with different properties. Ex: wood burns to form ash and smoke Chemical properties cannot be observed with your senses. Chemical properties aren’t as easy to observe as physical properties. Examples of chemical properties: flammability and reactivity

Characteristic Properties o o The properties that are most useful in identifying a substance are its characteristic properties. Remember the difference between physical and chemical properties. Physical properties can be observed! (with your eyes!) IDENTITY OF SUBSTANCE DOES NOT CHANGE! You can observe chemical properties only in situations in which the identity of the substance could change.

Some Characteristic Properties o o o So which properties allow us the identify unknown substances? Density p. H Solubility Melting and boiling points Etc…

Spotlight on Density o o o Density is a very helpful physical property. Density = mass per unit of volume or Density = mass/volume Density is an excellent help in identifying substances because each substance has its own density.

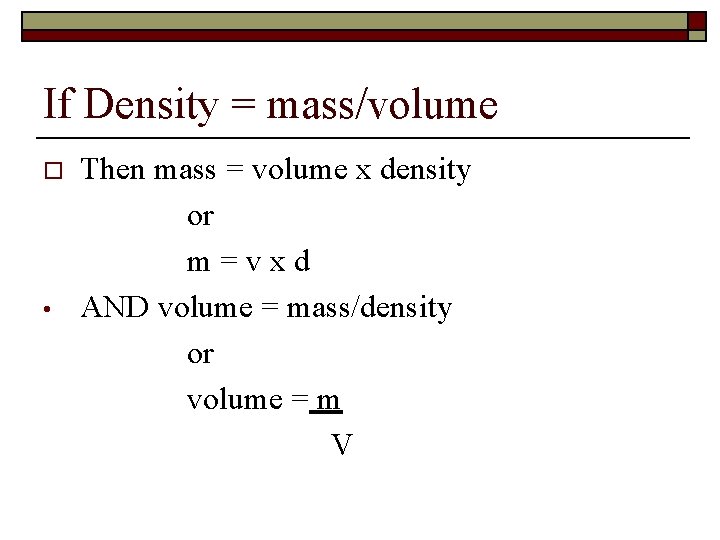
If Density = mass/volume o • Then mass = volume x density or m=vxd AND volume = mass/density or volume = m V

Spotlight on Solubility o o o Solubility is the maximum amount of solute that can be dissolved in a given amount of solvent Can be measured in g/L Solubility depends on temperature Solubility of gas in water

Other Characteristic Properties o Solids: electrical conductivity & flame test o Liquids: reaction to litmus & cobalt chloride paper o Gases: reaction with glowing splint, burning splint & limewater

Gas Tests o Oxygen o Hydrogen o Carbon dioxide

Physical Changes o o o A physical change is a change that affects one or more physical properties of a substance. Physical changes do not form new substances! EX: ice melting or sugar dissolving Physical changes are easy to undo.

Chemical Changes o o o A chemical change occurs when one or more substances are changed into entirely new substances with different properties. You can observe chemical properties only when a chemical change might occur! Examples of chem. changes: baking a cake rusting

Clues to chemical changes o Color change Fizzing or bubbling (gas production) Not to be confused with boiling! Heat Production of light, sound, or odor. o Chemical changes are not usually reversible! o o o
 Chemical property of water
Chemical property of water Descriptive matter
Descriptive matter Alleluia hat len nguoi oi
Alleluia hat len nguoi oi điện thế nghỉ
điện thế nghỉ Fecboak
Fecboak Một số thể thơ truyền thống
Một số thể thơ truyền thống Sơ đồ cơ thể người
Sơ đồ cơ thể người Công thức tiính động năng
Công thức tiính động năng Các số nguyên tố
Các số nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Tư thế ngồi viết
Tư thế ngồi viết V. c c
V. c c Gấu đi như thế nào
Gấu đi như thế nào Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật













































