Properties and Changes in Matter PROPERTIES OF MATTER







- Slides: 12

Properties and Changes in Matter

PROPERTIES OF MATTER

Physical Properties • Can be observed or viewed without changing the sample’s composition – Example: density, color, odor, state of matter, boiling point, melting point, shape – Use your senses

Properties Extensive • Properties that depend on the amount of substance present • Example: Volume, mass Intensive • Properties that are independent of the amount of substance present • Can be used to identify a substance • Example: density, boiling point, temperature

Chemical Properties • How a substance interacts with another substance (ability or inability) • Example: iron can react with oxygen, carbon dioxide is not flammable

CHANGES IN MATTER

Physical Changes • Alter a substance without changing the composition • Example: bend, grind, crumple, split, phase change

Chemical Changes • Process in which a substance(s) is changed into one or more new substances • Example: combust, ferment, burn, rot, tarnish • Also known as a chemical reaction

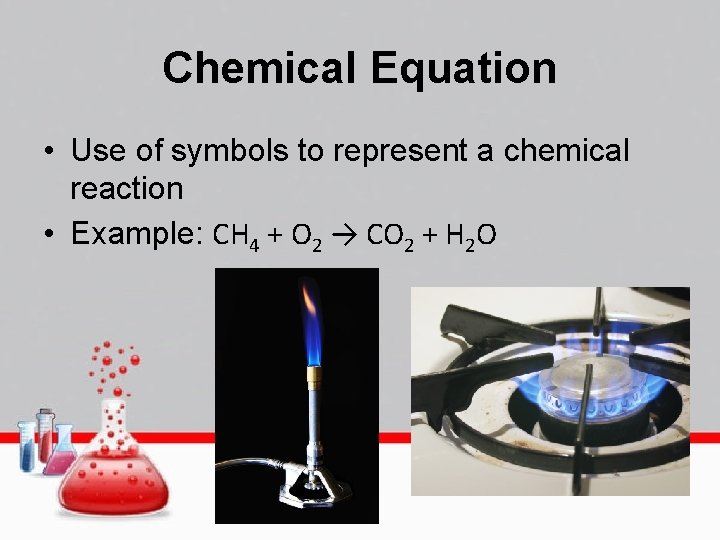
Chemical Equation • Use of symbols to represent a chemical reaction • Example: CH 4 + O 2 → CO 2 + H 2 O

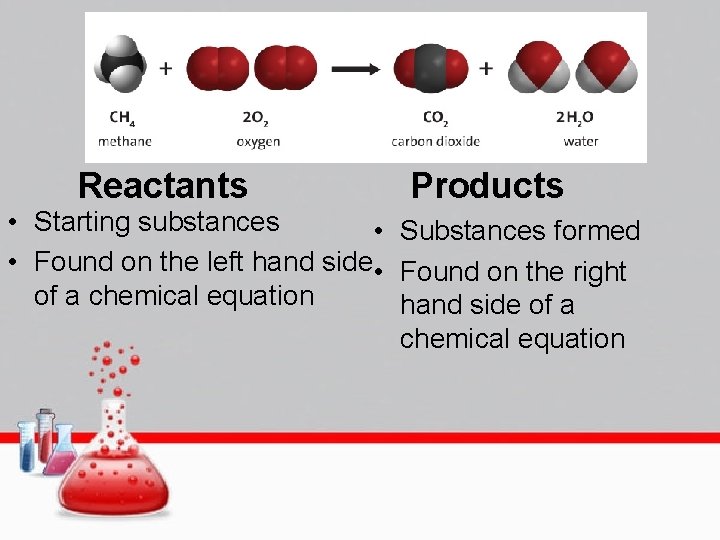
Reactants Products • Starting substances • Substances formed • Found on the left hand side • Found on the right of a chemical equation hand side of a chemical equation

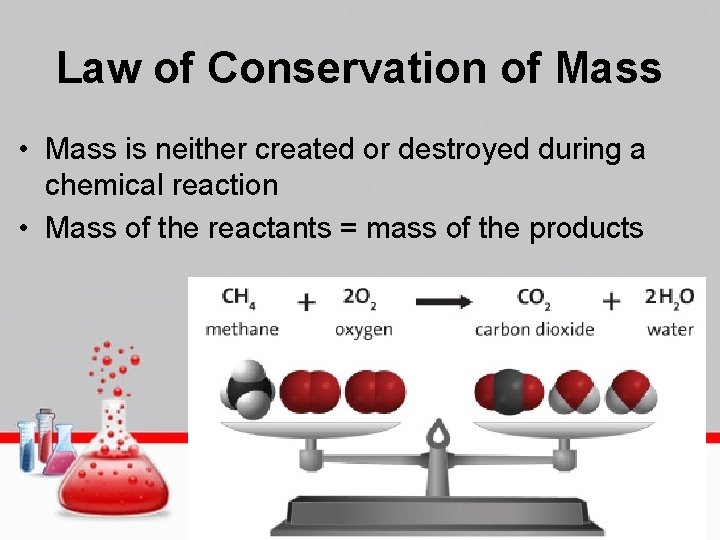
Law of Conservation of Mass • Mass is neither created or destroyed during a chemical reaction • Mass of the reactants = mass of the products

Chemical Reaction Signs • • • Bubbling (gas production) Color change Precipitate forms (solid formation) Energy change (temperature change) The only way to know for sure is to check the composition before and after!!
 Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Big idea 9 changes in matter
Big idea 9 changes in matter Elizabeth mulroney
Elizabeth mulroney What is a physical change
What is a physical change Chemistry matter and its changes
Chemistry matter and its changes Eating food physical or chemical change
Eating food physical or chemical change Chemical properties and changes lesson 4
Chemical properties and changes lesson 4 Physical change
Physical change Phases changes of matter
Phases changes of matter Change in state of matter
Change in state of matter Two types of changes
Two types of changes Diffusion vs effusion
Diffusion vs effusion























