Matter Classification of Matter Methods for Classifying Matter









- Slides: 16

Matter

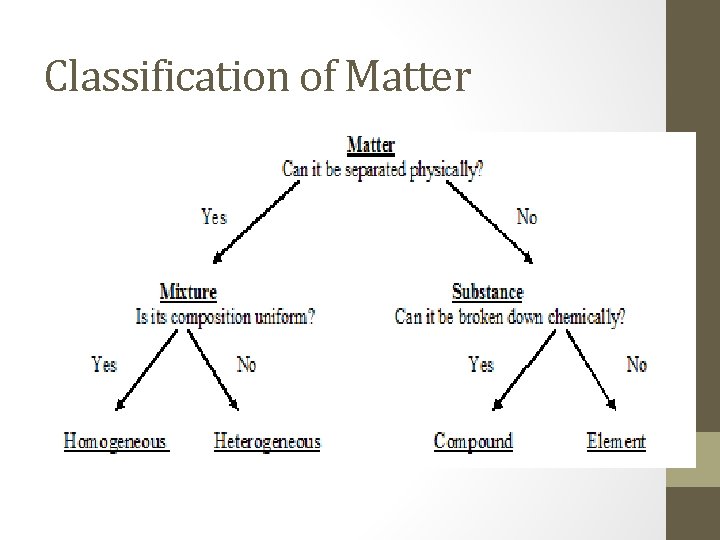
Classification of Matter

Methods for Classifying Matter 1. If the substance is listed on the periodic table, then it is an element. 2. If you put an “and” in the substance’s name, then it is a mixture. example: peanut butter and jelly 3. If the substance has more than one type of atom, then it is a compound.

Classification of Matter Substances are either elements or compounds. Elements cannot be broken down by chemical reactions. Compounds can be broken down to simpler compounds or elements by a chemical change. Example: Water can be broken into hydrogen and oxygen gas with electrolysis.

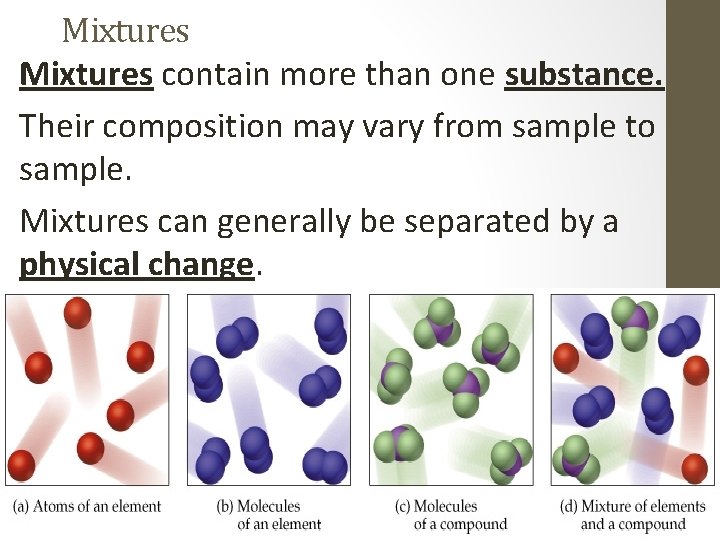
Mixtures contain more than one substance. Their composition may vary from sample to sample. Mixtures can generally be separated by a physical change.

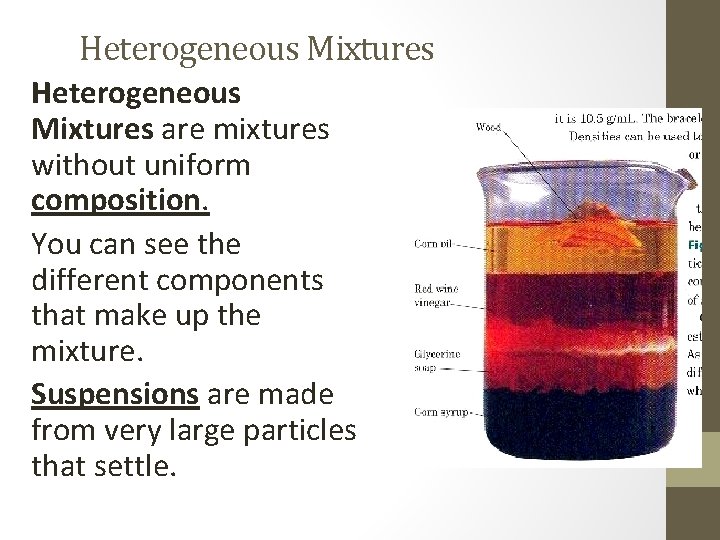
Heterogeneous Mixtures are mixtures without uniform composition. You can see the different components that make up the mixture. Suspensions are made from very large particles that settle.

Homogeneous Mixtures Homogeneous mixtures or solutions are mixtures with uniform composition. Colloids are made with larger particles than a solution.

Physical and Chemical Properties and Changes

Physical and Chemical Properties • A physical property is a characteristic of a substance that can be observed or measured without changing the substance. • These can be identified by your senses. • Examples of physical properties are melting/freezing point, density, size, color, tensile strength, and many others. • A chemical property is a characteristic of a substance that is only observed by changing the substance into something new. • This describes how a substance behaves when it changes into a new substance. • Example of chemical properties are flammability, reactivity, toxicity, heat of combustion, and others.

Physical Changes • All matter can undergo changes, but energy is needed for changes. • A physical change is one in which matter only changes its physical form. It is the same substance, just has a different appearance. • A physical change can occur when a substance changes it’s state of matter (solid, liquid, gas). • Other appearance changes tearing paper, chopping wood, dissolving sugar or salt in water are all physical changes.

Chemical Changes • Chemical changes occur when a substance changes into a completely new substance. This will involve a chemical reaction like photosynthesis. • Indicators of a chemical change are: • Permanent color change • Change in odor • Change in temperature (exothermic or endothermic) • Light produced • Precipitate (insoluble solid made by reaction) formed • Gas being produced

States of Matter Solids, Liquids, and Gases

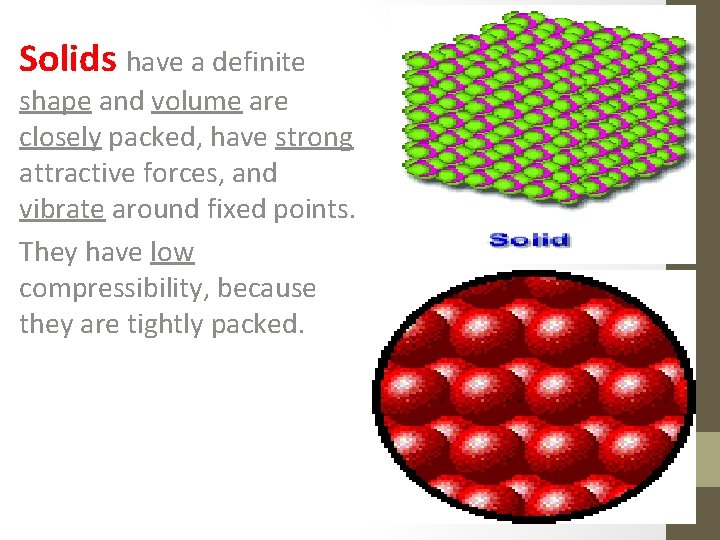
Solids have a definite shape and volume are closely packed, have strong attractive forces, and vibrate around fixed points. They have low compressibility, because they are tightly packed.

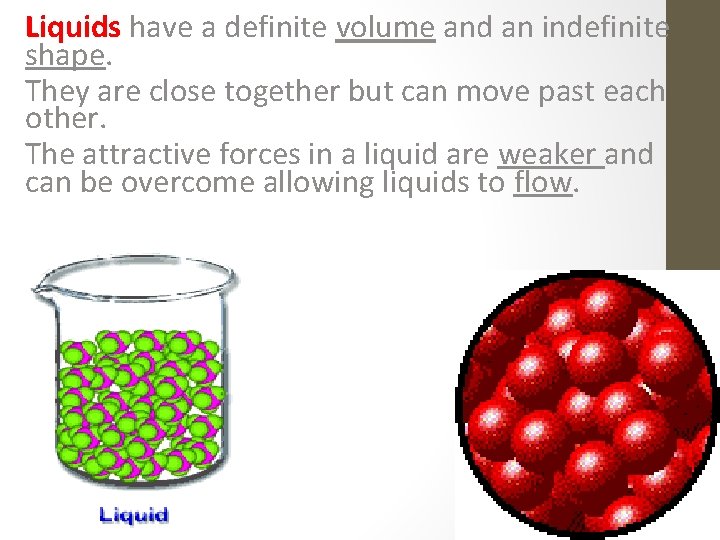
Liquids have a definite volume and an indefinite shape. They are close together but can move past each other. The attractive forces in a liquid are weaker and can be overcome allowing liquids to flow.

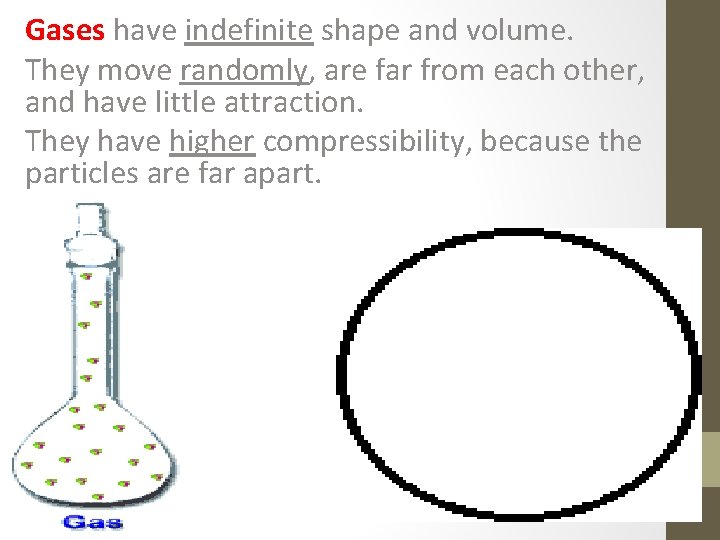
Gases have indefinite shape and volume. They move randomly, are far from each other, and have little attraction. They have higher compressibility, because the particles are far apart.

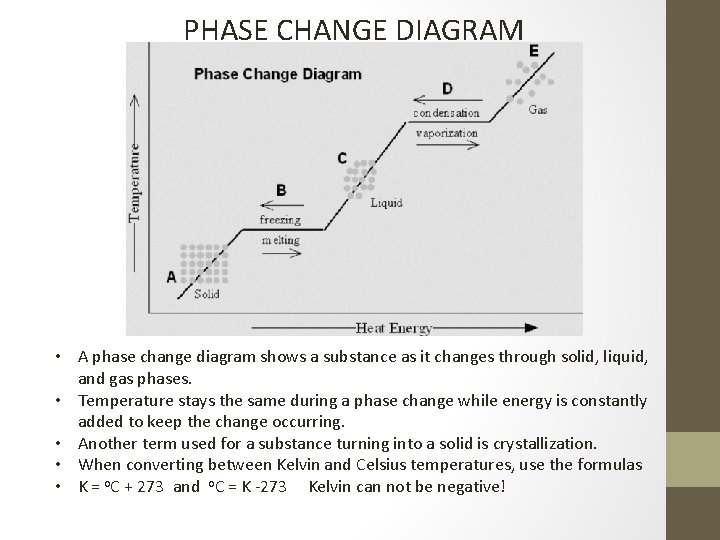
PHASE CHANGE DIAGRAM • A phase change diagram shows a substance as it changes through solid, liquid, and gas phases. • Temperature stays the same during a phase change while energy is constantly added to keep the change occurring. • Another term used for a substance turning into a solid is crystallization. • When converting between Kelvin and Celsius temperatures, use the formulas • K = o. C + 273 and o. C = K -273 Kelvin can not be negative!