MATTER Classification of Matter Composition of Matter 10




















- Slides: 25

MATTER Classification of Matter Composition of Matter

10. 1 The Nature of Matter l Matter is a term used to describe anything that has mass and takes up space. l Greek philosophers Democritus and Leucippus proposed that matter is made of tiny particles called atoms. l Atoms were an idea that few believed. l The first evidence was called Brownian motion for Robert Brown, who first noticed the jerky motion of tiny particles.

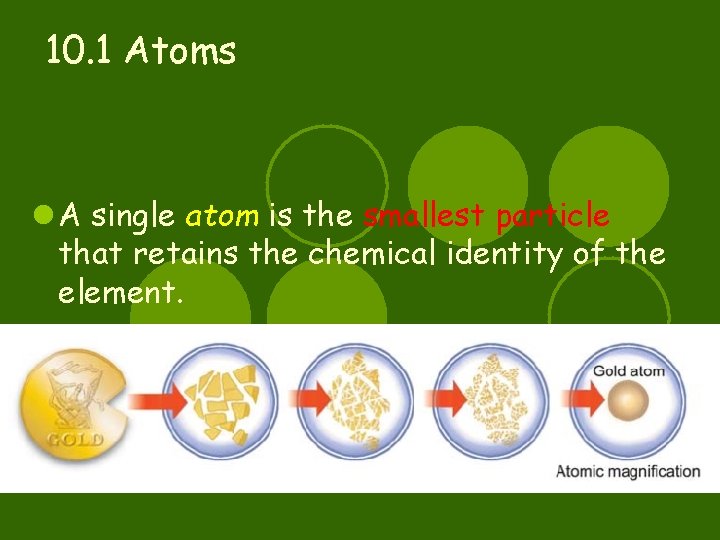
10. 1 Atoms l A single atom is the smallest particle that retains the chemical identity of the element.

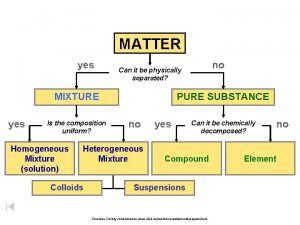
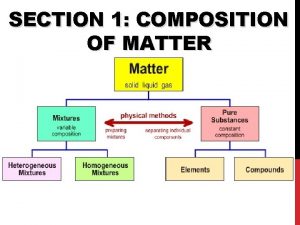
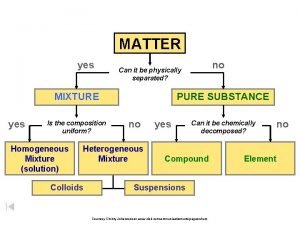
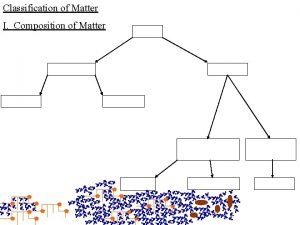
Pure Substances l Matter is classified as substances or a mixture of substances. l A pure substance, type of matter with a fixed composition that cannot be separated by physical means. l A substance can be either an element or a compound.

Pure Substances l Element ¡matter composed of identical atoms (same identity) ¡EX: copper, gold, lead

Elements l About 90 elements are found on Earth. l More than 20 others have been made in laboratories, but most of these are unstable and exist only for short periods of time. l More on that later…

Compounds l Can you imagine yourself putting something made from a slivery metal and a greenish-yellow, poisonous gas on your food?

Compounds l Table salt is a chemical compound that fits this description. ¡Even though it looks like white crystals and adds flavor to food, its components—sodium and chlorine—are neither white nor salty.

l Compound ¡Atoms of two or more elements combined ¡properties differ from those of individual elements ¡EX: salt (Na. Cl), water, chalk

Mixtures o A mixture, such as the pizza or soft drink shown, is a material made up of two or more substances that can be easily separated by physical means.

Mixtures l Variable combination of 2 or more pure substances that can be separated by physical means

l Homogeneous Mixture (Solution) ¡even distribution of components ¡very small particles ¡particles never settle ¡EX: saline solution, fresh pickle juice, ¡ vinegar, soda

Heterogeneous Mixtures l Unlike compounds, mixtures do not always contain the same proportions of the substances that make them up. l A mixture in which different materials can be distinguished easily is called a heterogeneous mixture.

Mixtures l Heterogeneous Mixture ¡uneven distribution of components- mixture in which different materials can be easily distinguished ¡colloids and suspensions ¡EX: granite, permanent press fabric

Solution l Solution – homogeneous mixture of particles so small that they cannot even be seen with a microscope and will never settle to the bottom of their container l Ex. Vinegar, soda, hydrogen peroxide

Colloids l A Colloid is a type of mixture with particles that are larger than those in solutions but not heavy enough to settle out. l Ex. Milk (water & fat)

Mixtures l Colloid ¡Detecting colloids is sometimes difficult so shining a beam of light at colloid will make the light scatter – this scattering of light by a colloid is called the Tyndall effect.

Tyndall Effect A light beam is Scattered by the Colloid suspension On the left, but Passes invisibly Through the solution On the right.

Mixtures l Suspension ¡Heterogeneous mixture containing a liquid in which visible particles settle ¡EX: Italian salad dressing (oil, vinegar, and spices), a river delta, pond ¡Hint: if it needs shaking to mix, then it’s a suspension

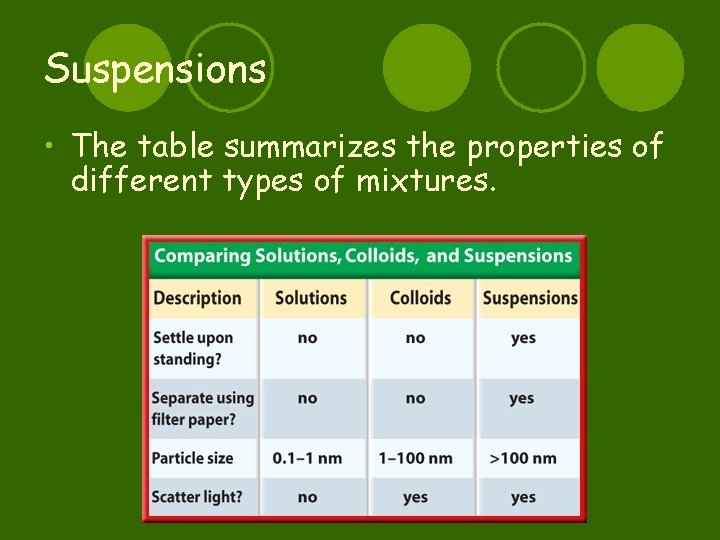
Suspensions • The table summarizes the properties of different types of mixtures.

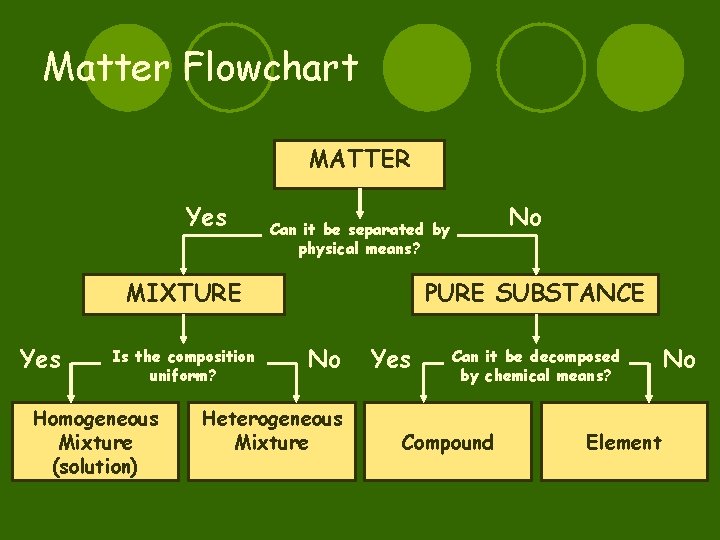
Matter Flowchart MATTER Yes MIXTURE Yes Is the composition uniform? Homogeneous Mixture (solution) No Can it be separated by physical means? PURE SUBSTANCE No Heterogeneous Mixture Yes Can it be decomposed by chemical means? Compound Element No


States of Matter l Matter – anything that has mass and takes up space l Kinetic theory – explains how particles in matter behave ¡All matter is composed of particles ¡Particles are in constant, random motion ¡Particles collide with each other and walls of their container

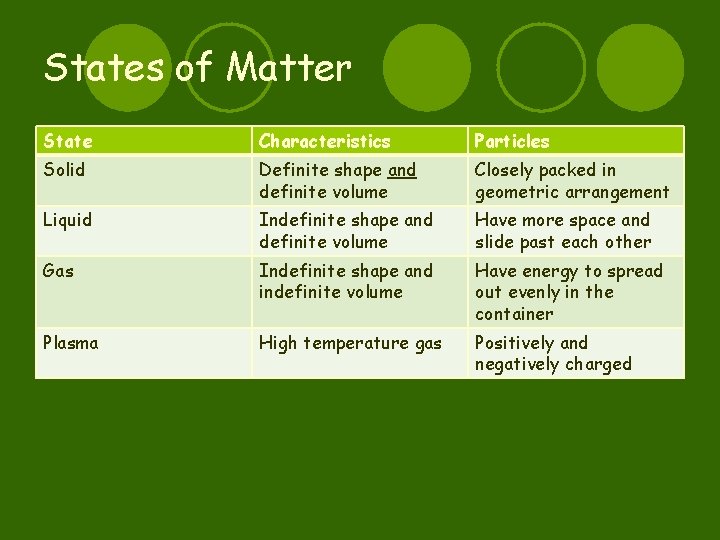
States of Matter State Characteristics Particles Solid Definite shape and definite volume Closely packed in geometric arrangement Liquid Indefinite shape and definite volume Have more space and slide past each other Gas Indefinite shape and indefinite volume Have energy to spread out evenly in the container Plasma High temperature gas Positively and negatively charged

l The state of a sample of matter depends on temperature. ¡Temperature – related to the average kinetic energy of an object’s atoms and molecules. ¡Thermal expansion – increase in the size of a substance when the temperature increases and contracts when cooled ¡**exception to the rule: water – when cooled it expands
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Composition of matter section 1
Composition of matter section 1 Is the composition uniform
Is the composition uniform Flowchart of matter
Flowchart of matter The study of composition structure and properties
The study of composition structure and properties What is dissolution
What is dissolution Compounds vs mixtures
Compounds vs mixtures What is a matter flow chart
What is a matter flow chart Composition of matter chapter 9
Composition of matter chapter 9 Section 1 composition of matter
Section 1 composition of matter Physical change concept map
Physical change concept map Composition of matter flow chart
Composition of matter flow chart Composition of matter notes
Composition of matter notes Liquid information
Liquid information Mixture
Mixture Protein elements
Protein elements Grey matter of nervous system
Grey matter of nervous system Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Optic tract
Optic tract Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Ecological succession
Ecological succession Classification and properties of matter
Classification and properties of matter Worksheet classification of matter
Worksheet classification of matter What is classification of matter
What is classification of matter