SECTION 1 COMPOSITION OF MATTER Warmup Think of
















- Slides: 23

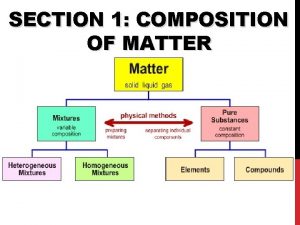
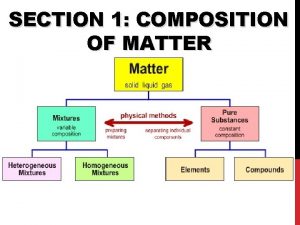
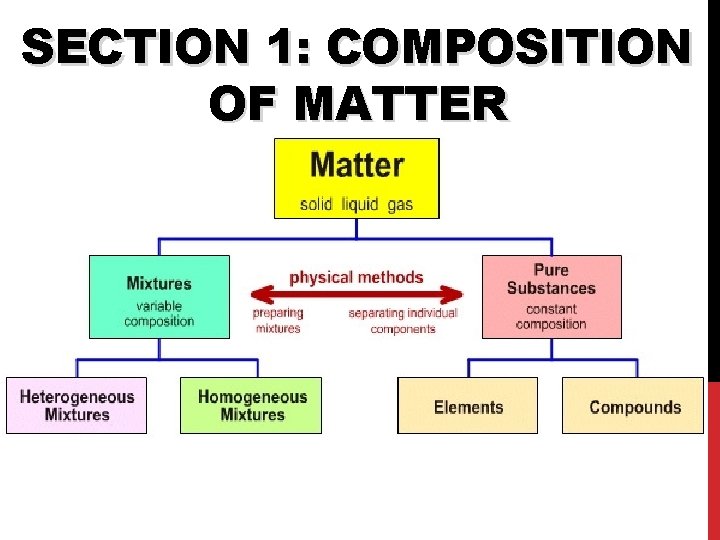
SECTION 1: COMPOSITION OF MATTER

Warm-up: • Think of that air you are breathing in right now. • What are some components of air? • Why might air be referred to as a mixture?

Learning Goals • Define substances and mixtures. • Identify elements and compounds. • Compare and contrast solutions, colloids, and suspensions.

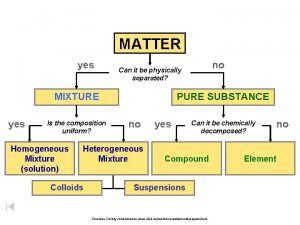
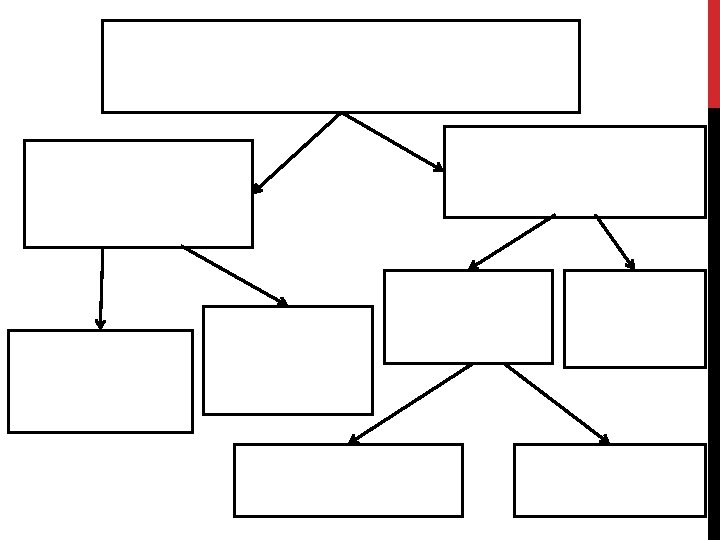
Matter • Matter: anything that has mass and takes up space • All matter can be divided into substances and mixtures.

Substances • Substances: type of matter with a fixed composition • can be either elements or compounds.

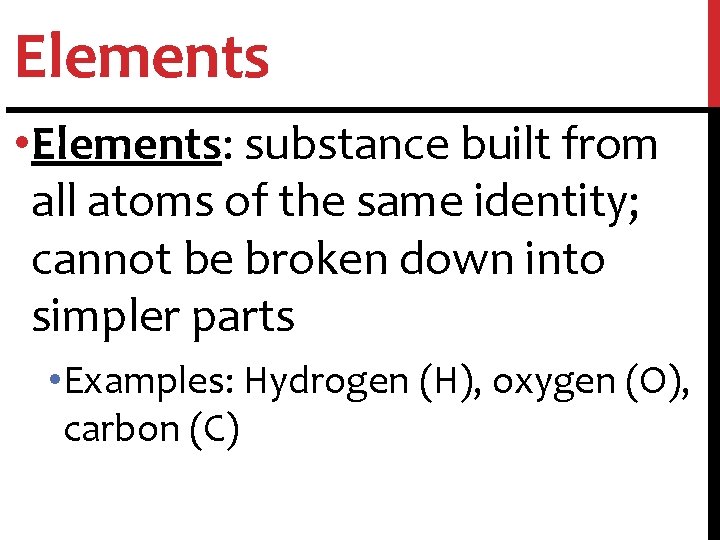
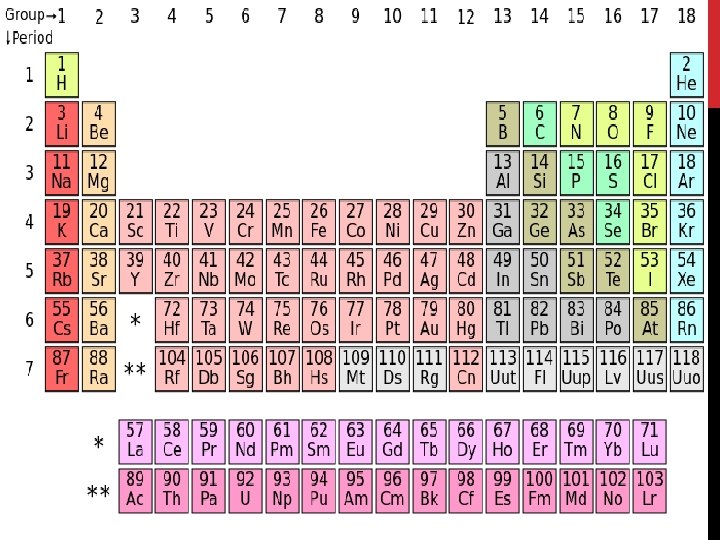
Elements • Elements: substance built from all atoms of the same identity; cannot be broken down into simpler parts • Examples: Hydrogen (H), oxygen (O), carbon (C)

Elements • All elements are listed on the periodic table. • There about 90 naturally occurring elements. • Approximately 20 have been made in laboratories.


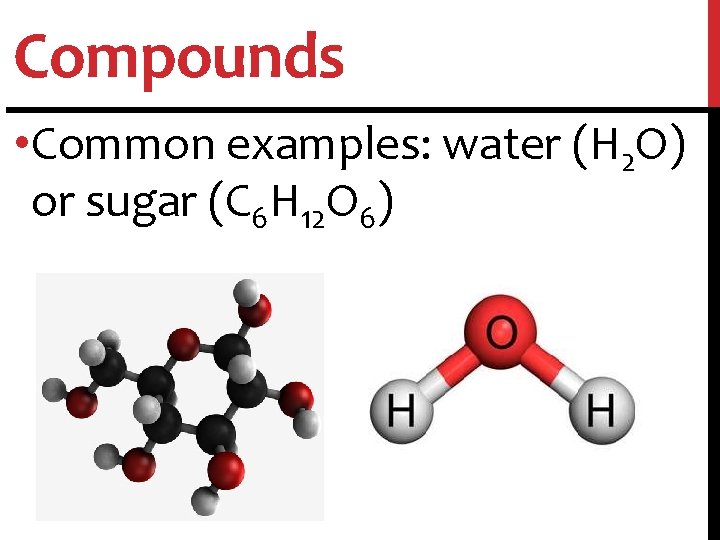
Compounds • Compounds: substance in which two or more atoms are combined in a fixed proportion

Compounds • Common examples: water (H 2 O) or sugar (C 6 H 12 O 6)

Mixtures • Mixture: material made of two or more substances that can be separated by physical means • Mixtures can be heterogeneous or homogeneous

Heterogeneous Mixtures • Heterogeneous mixture: a mixture in which the different materials can be distinguished easily

Heterogeneous Mixtures • Examples: salad, granite, dry soup mix

Homogeneous Mixtures • Homogeneous mixture: contains two or more gaseous, liquid, or solid substances blended evenly throughout • Examples: vinegar (acetic acid and water), iced tea, fog, smoke

Homogenous Mixtures • Homogeneous mixtures can be described as solutions or colloids

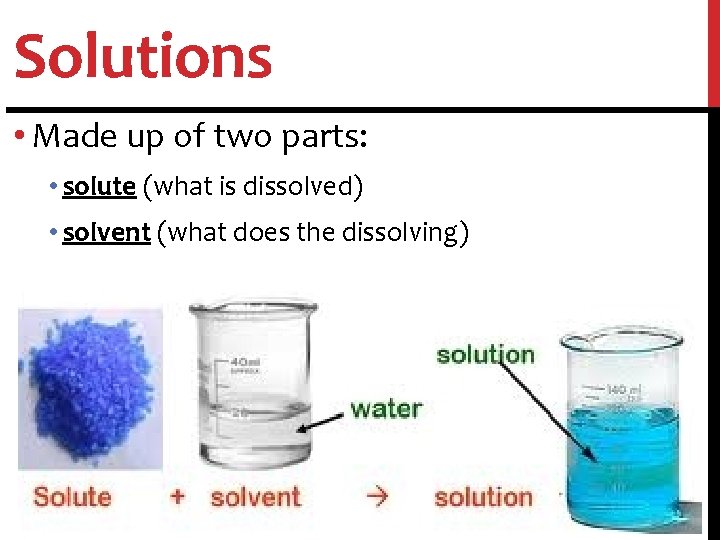
Solutions • Solution: homogeneous mixture with particles so small that they cannot be seen with a microscope and will never settle to the bottom of the container • Examples: lemonade, salt water

Solutions • Made up of two parts: • solute (what is dissolved) • solvent (what does the dissolving)

Colloids • Colloids: type of mixture with particles that are larger than those in solutions but are not heavy enough to settle out • Examples: paint, fog, smoke

Colloids • Colloids are detected using the Tyndall effect where you pass light through the substance. The particles in the colloid will scatter the light.

Suspensions • Some mixtures are neither solutions nor colloids • Suspension: heterogeneous mixture containing a liquid in which visible particles settle. • Examples: pond water, Italian dressing

Suspensions


Check-in: • Why might it be easy to confuse a compound a homogeneous mixture?
 Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Think big think fast
Think big think fast What is dissolution
What is dissolution Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Warmup ratio
Warmup ratio Warmup 65
Warmup 65 Gmass warmup
Gmass warmup Stratified warmup
Stratified warmup Pyramid warmup
Pyramid warmup Mind rhyming words
Mind rhyming words Exponent properties
Exponent properties Java warmup
Java warmup Define:warmup
Define:warmup Ethos warmup
Ethos warmup Tinman calculator
Tinman calculator Warmup 65
Warmup 65 Warmup end
Warmup end A little bird by aileen fisher
A little bird by aileen fisher Think family ni
Think family ni Matter with uniform compositions.
Matter with uniform compositions. Matter flow chart
Matter flow chart