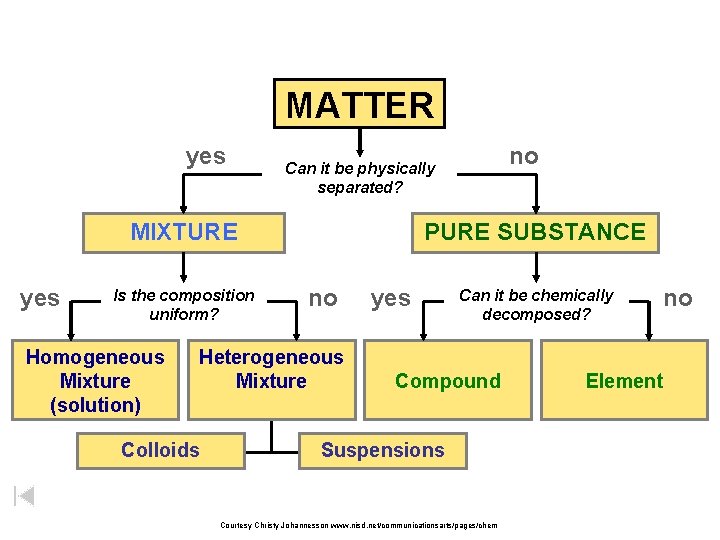
MATTER yes MIXTURE yes Is the composition uniform







- Slides: 30

MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem no Element

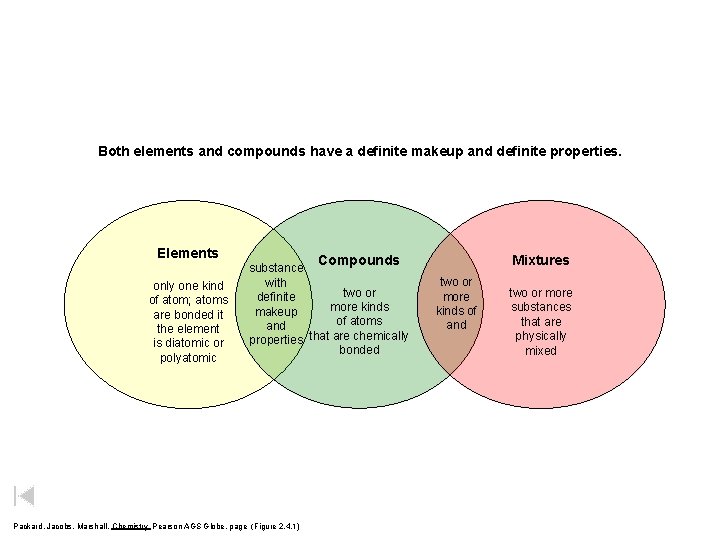
Both elements and compounds have a definite makeup and definite properties. Elements only one kind of atom; atoms are bonded it the element is diatomic or polyatomic Compounds substance with two or definite more kinds makeup of atoms and that are chemically properties bonded Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page (Figure 2. 4. 1) Mixtures two or more kinds of and two or more substances that are physically mixed

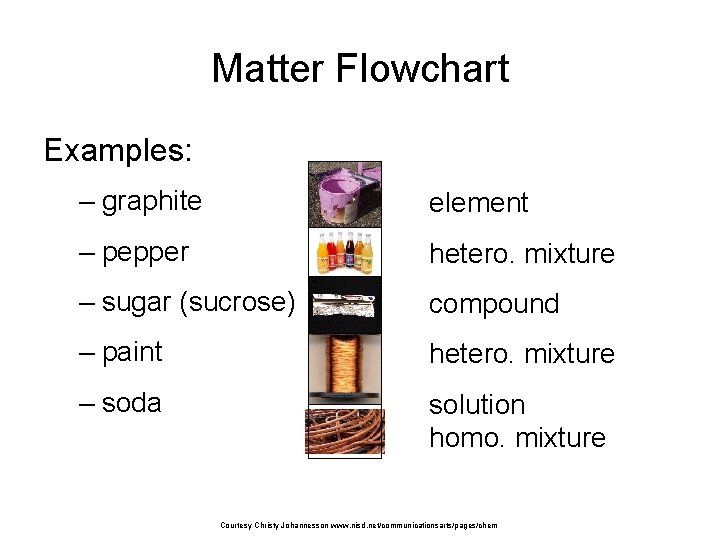
Matter Flowchart Examples: – graphite element – pepper hetero. mixture – sugar (sucrose) compound – paint hetero. mixture – soda solution homo. mixture Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Pure Substances Element – composed of identical atoms – EX: copper wire, aluminum foil Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

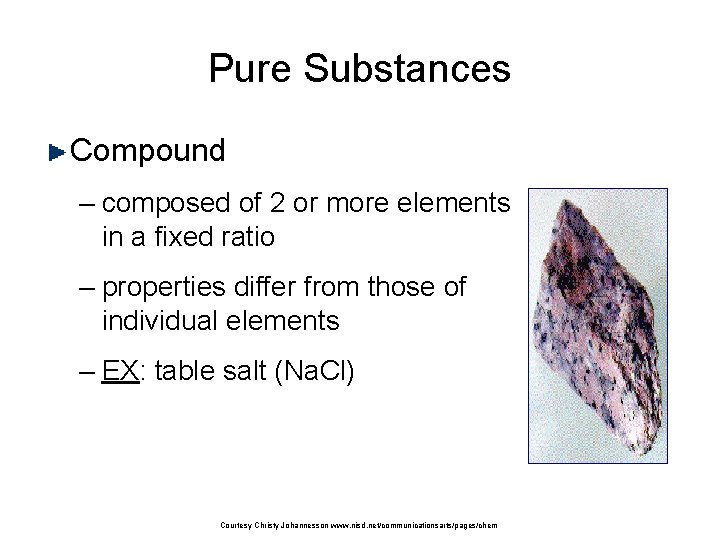
Pure Substances Compound – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl) Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Pure Substances Law of Definite Composition – A given compound always contains the same, fixed ratio of elements. Law of Multiple Proportions – Elements can combine in different ratios to form different compounds. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Pure Substances For example… Carbon, C Oxygen, O Carbon monoxide, CO Carbon dioxide, CO 2 Two different compounds, each has a definite composition. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Mixtures Variable combination of two or more pure substances. Heterogeneous Homogeneous Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Mixtures Solution – homogeneous – very small particles – no Tyndall effect Tyndall Effect – particles don’t settle – EX: rubbing alcohol Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Mixtures Colloid – heterogeneous – medium-sized particles – Tyndall effect – particles don’t settle – EX: milk Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Mixtures Suspension – heterogeneous – large particles – Tyndall effect – particles settle – EX: fresh-squeezed lemonade Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Mixtures Examples: – mayonnaise colloid – muddy water suspension – fog colloid – saltwater solution – Italian salad dressing suspension Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

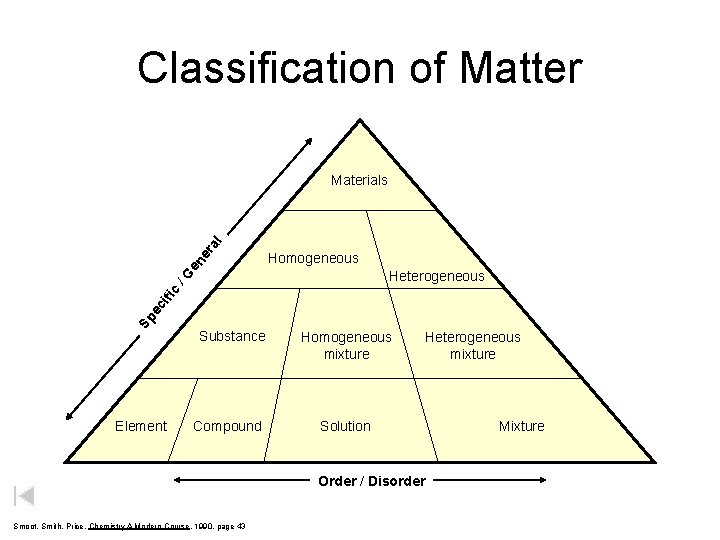
Classification of Matter Homogeneous Heterogeneous Sp ec ifi c /G en er a l Materials Element Substance Compound Homogeneous mixture Heterogeneous mixture Solution Order / Disorder Smoot, Smith, Price, Chemistry A Modern Course, 1990, page 43 Mixture

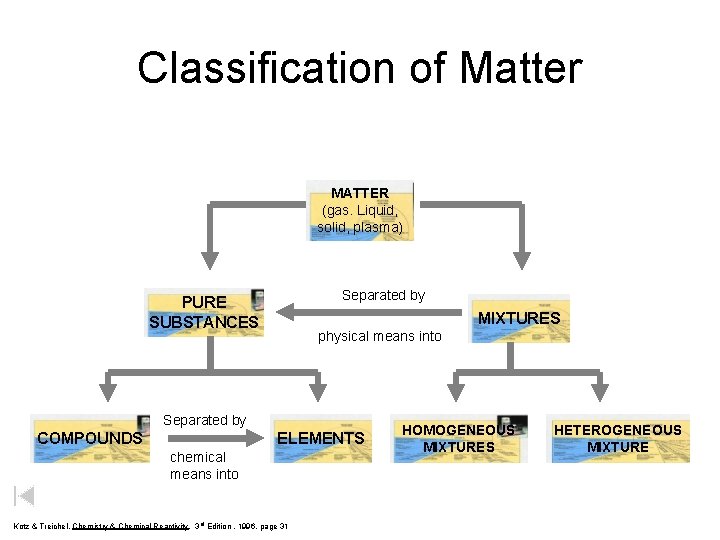
Classification of Matter MATTER (gas. Liquid, solid, plasma) Separated by PURE SUBSTANCES MIXTURES physical means into Separated by COMPOUNDS ELEMENTS chemical means into Kotz & Treichel, Chemistry & Chemical Reactivity, 3 rd Edition , 1996, page 31 HOMOGENEOUS MIXTURES HETEROGENEOUS MIXTURE

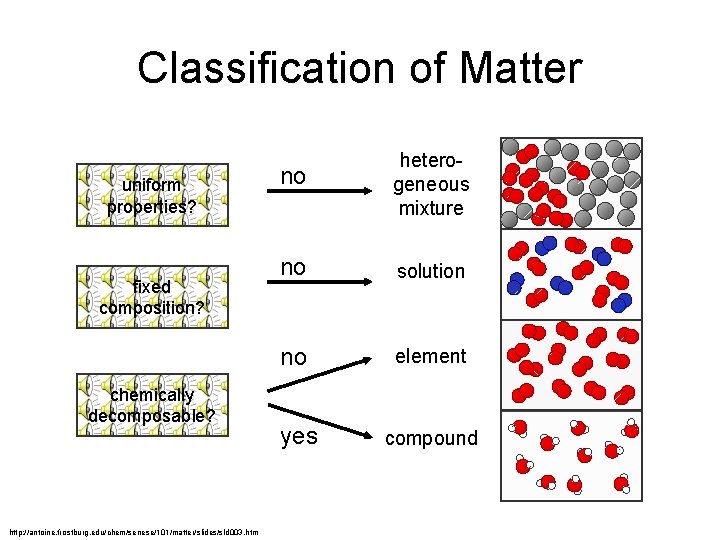
Classification of Matter uniform properties? fixed composition? chemically decomposable? http: //antoine. frostburg. edu/chem/senese/101/matter/slides/sld 003. htm no heterogeneous mixture no solution no element yes compound

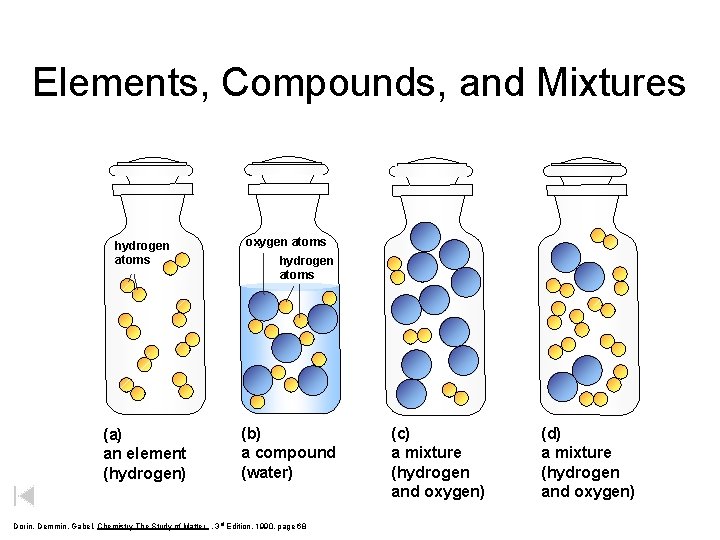
Elements, Compounds, and Mixtures hydrogen atoms oxygen atoms (a) an element (hydrogen) (b) a compound (water) hydrogen atoms Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 (c) a mixture (hydrogen and oxygen) (d) a mixture (hydrogen and oxygen)

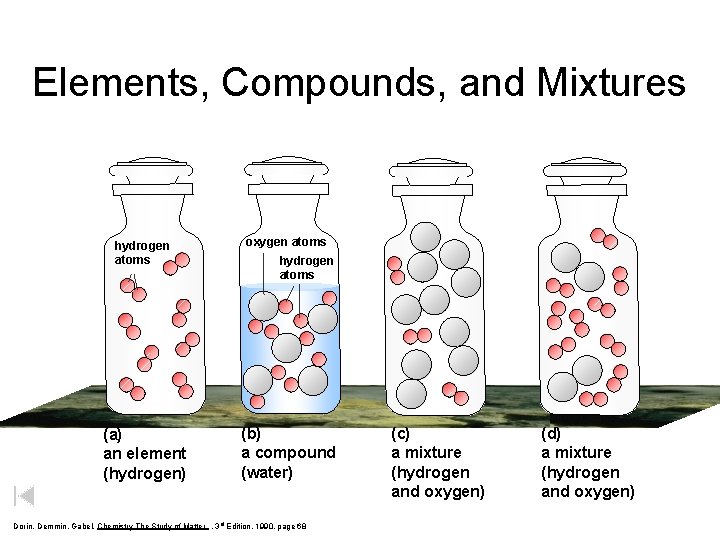
Elements, Compounds, and Mixtures hydrogen atoms oxygen atoms (a) an element (hydrogen) (b) a compound (water) hydrogen atoms Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 (c) a mixture (hydrogen and oxygen) (d) a mixture (hydrogen and oxygen)

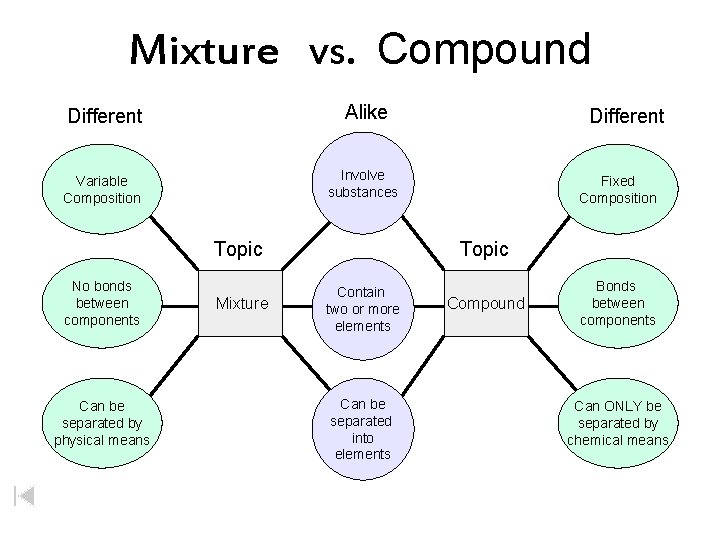
Mixture vs. Compound Different Alike Variable Composition Involve substances Topic No bonds between components Can be separated by physical means Mixture Different Fixed Composition Topic Contain two or more elements Can be separated into elements Compound Bonds between components Can ONLY be separated by chemical means

Compounds vs. Mixtures • Compounds have properties that are uniquely different from the elements from which they are made. – A formula can always be written for a compound – e. g. Na. Cl Na + Cl 2 • Mixtures retain their individual properties. – e. g. Salt water is salty and wet

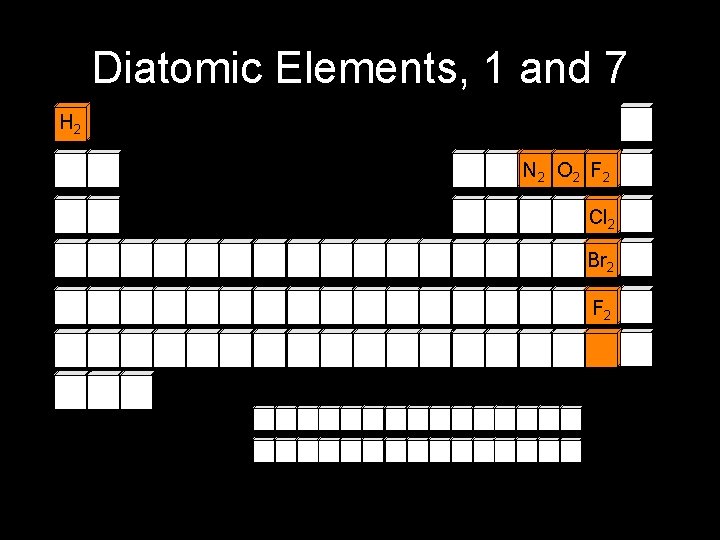
Diatomic Elements, 1 and 7 H 2 N 2 O 2 F 2 Cl 2 Br 2 F 2

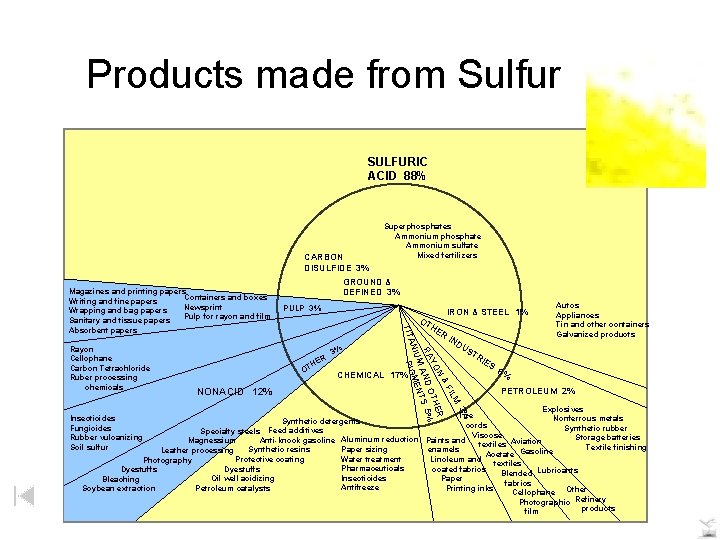
Products made from Sulfur SULFURIC ACID 88% CARBON DISULFIDE 3% PULP 3% ER TH O NONACID 12% 3% IRON & STEEL 1% OT HE R IN DU 3% M FIL & N ER YO OTH RA AND S 5% ENT IUM AN PIGM Rayon Cellophane Carbon Tetrachloride Ruber processing chemicals GROUND & DEFINED 3% TIT Magazines and printing papers Containers and boxes Writing and fine papers Newsprint Wrapping and bag papers Pulp for rayon and film Sanitary and tissue papers Absorbent papers Superphosphates Ammonium phosphate Ammonium sulfate Mixed fertilizers CHEMICAL 17% ST Autos Appliances Tin and other containers Galvanized products RI ES 6% PETROLEUM 2% Explosives Tire Nonferrous metals Synthetic detergents cords Synthetic rubber Specialty steels Feed additives Viscose Storage batteries Anti-knock gasoline Aluminum reduction Paints and Magnessium textiles Aviation Textile finishing Paper sizing enamels Synthetic resins Leather processing Gasoline Acetate Water treatment Linoleum and Protective coating Photography textiles Pharmaceuticals coated fabrics Dyestuffs Blended Lubricants Insecticides Paper Oil well acidizing Bleaching fabrics Antifreeze Printing inks Petroleum catalysts Soybean extraction Cellophane Other Photographic Refinery products film Insecticides Fungicides Rubber vulcanizing Soil sulfur

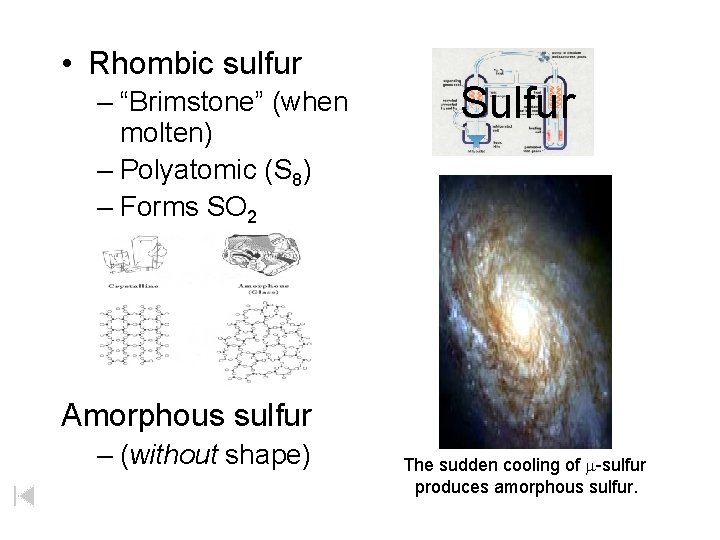
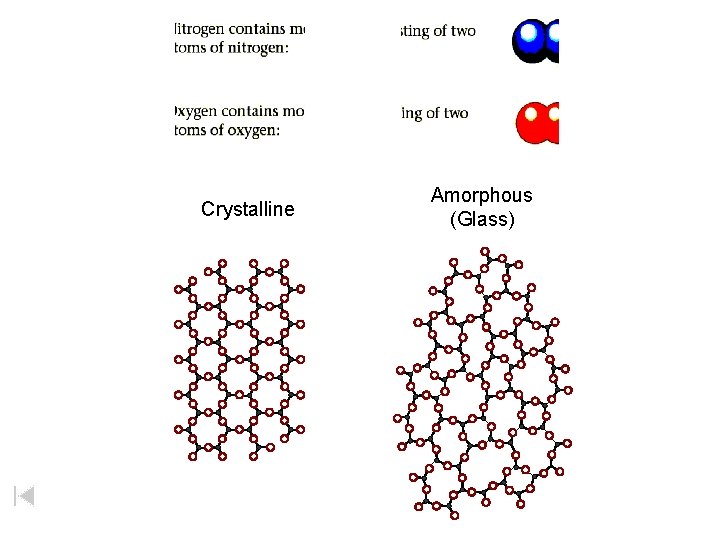
• Rhombic sulfur – “Brimstone” (when molten) – Polyatomic (S 8) – Forms SO 2 Sulfur Amorphous sulfur – (without shape) The sudden cooling of m-sulfur produces amorphous sulfur.

Crystalline Amorphous (Glass)

The Haber Process

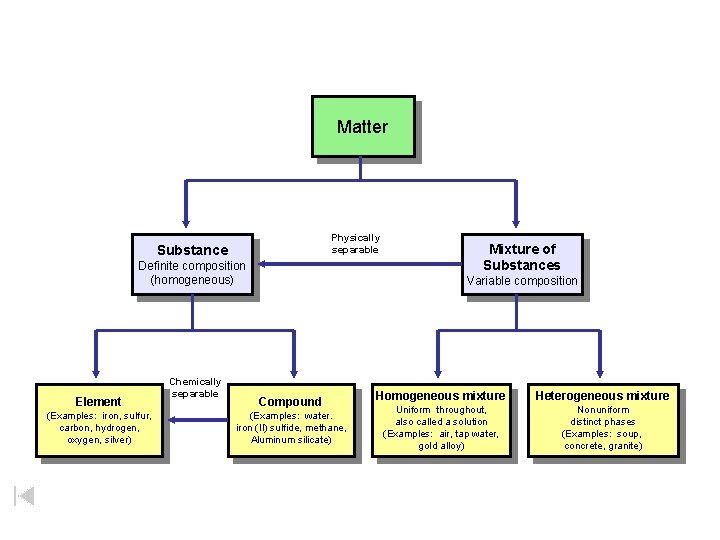
Substance Matter Physically separable Definite composition (homogeneous) Mixture of Substances Variable composition Chemically separable Homogeneous mixture Compound Uniform throughout, (Examples: iron, sulfur, (Examples: water. also called a solution carbon, hydrogen, iron (II) sulfide, methane, (Examples: air, tap water, oxygen, silver) Aluminum silicate) gold alloy) Element Heterogeneous mixture Nonuniform distinct phases (Examples: soup, concrete, granite)

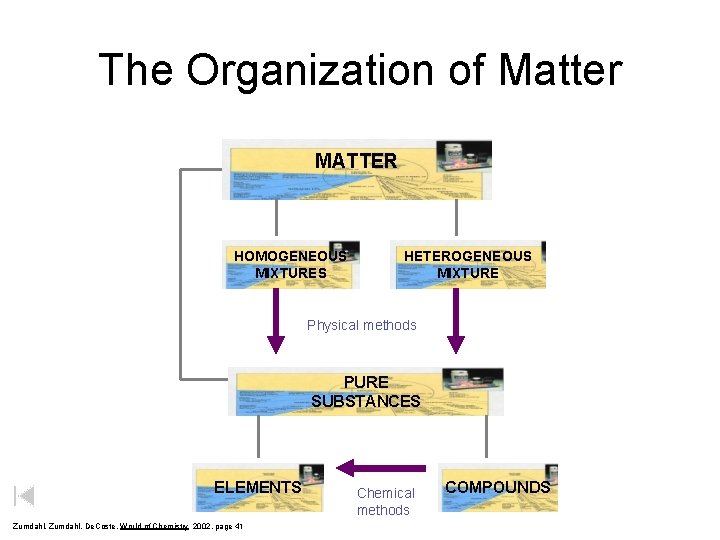
The Organization of Matter MATTER HOMOGENEOUS MIXTURES HETEROGENEOUS MIXTURE Physical methods PURE SUBSTANCES ELEMENTS Zumdahl, De. Coste, World of Chemistry 2002, page 41 Chemical methods COMPOUNDS

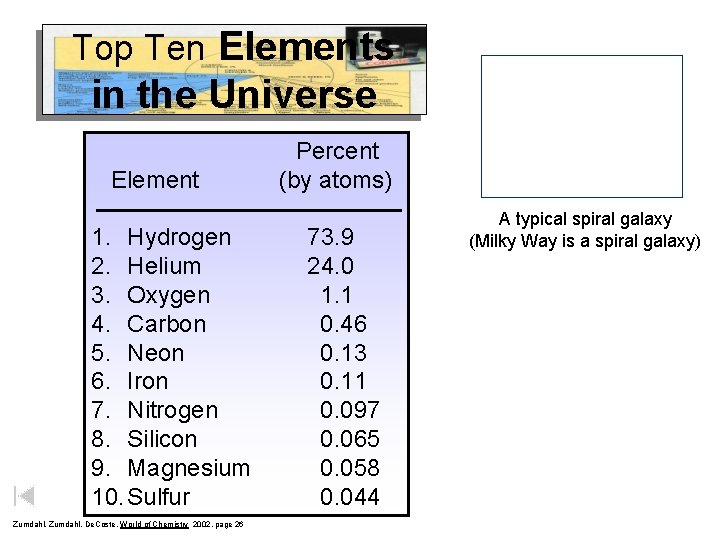
Top Ten Elements in the Universe Element 1. Hydrogen 2. Helium 3. Oxygen 4. Carbon 5. Neon 6. Iron 7. Nitrogen 8. Silicon 9. Magnesium 10. Sulfur Zumdahl, De. Coste, World of Chemistry 2002, page 26 Percent (by atoms) 73. 9 24. 0 1. 1 0. 46 0. 13 0. 11 0. 097 0. 065 0. 058 0. 044 A typical spiral galaxy (Milky Way is a spiral galaxy)

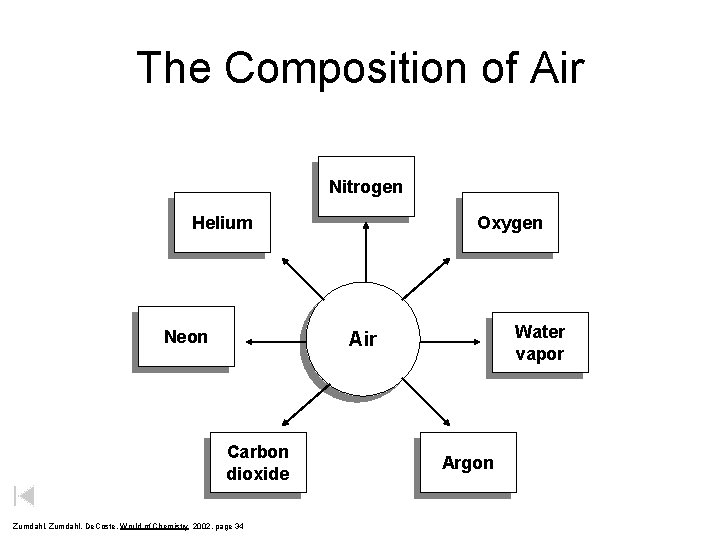
The Composition of Air Nitrogen Helium Neon Oxygen Water vapor Air Carbon dioxide Zumdahl, De. Coste, World of Chemistry 2002, page 34 Argon

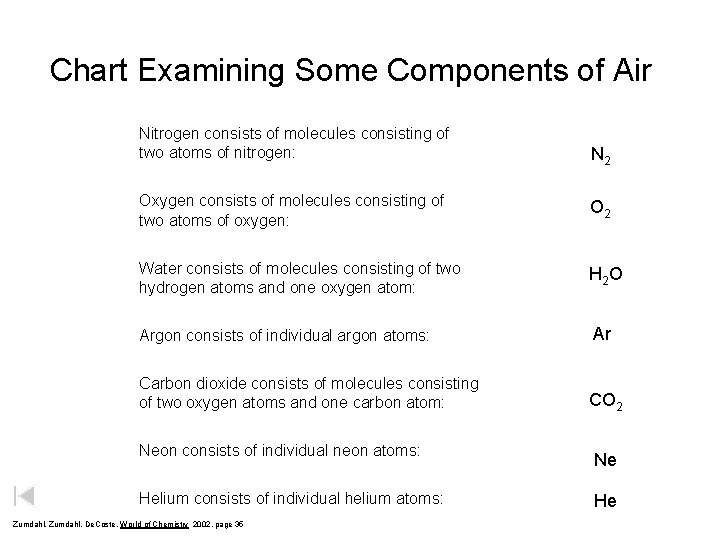
Chart Examining Some Components of Air Nitrogen consists of molecules consisting of two atoms of nitrogen: N 2 Oxygen consists of molecules consisting of two atoms of oxygen: O 2 Water consists of molecules consisting of two hydrogen atoms and one oxygen atom: H 2 O Argon consists of individual argon atoms: Ar Carbon dioxide consists of molecules consisting of two oxygen atoms and one carbon atom: CO 2 Neon consists of individual neon atoms: Helium consists of individual helium atoms: Zumdahl, De. Coste, World of Chemistry 2002, page 35 Ne He

Reviewing Concepts Classifying Matter • Why does every sample of a given substance have the same properties? • Explain why the composition of an element is fixed. • Describe the composition of a compound. • Why can the properties of a mixture vary? • On what basis can mixtures be classified as solutions, suspensions, or colloids?