General information Dr Kyriacos Kyriacou Biochemistry BSc UK

General information

Dr. Kyriacos Kyriacou • Biochemistry, BSc, UK, 1977 • Biochemistry, Ph. D, UK, 1982 • Spent 15 years in UK • At Frederick since 1991 2
Chemistry ACHM 111 • 4 ECTS , lecture and lab • Lecture once a week Group 1: Mondays 6: 00 -9: 30 PM Group 2: Tuesdays 6: 00 -9: 30 PM • Laboratory: Later in the course 3
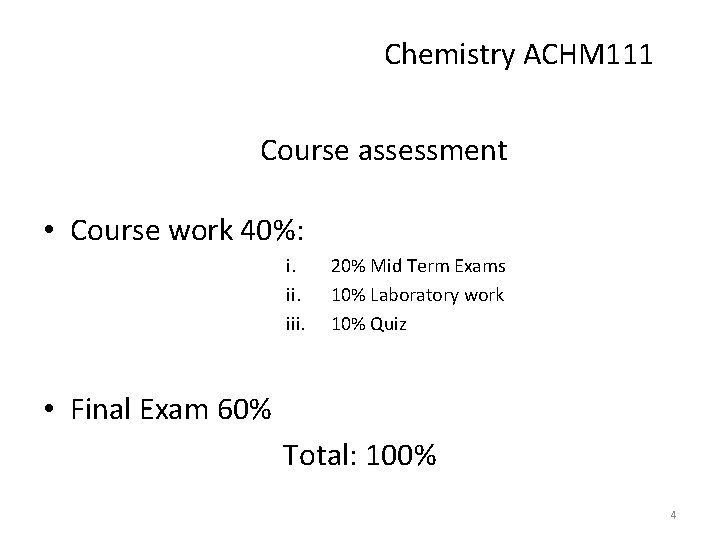
Chemistry ACHM 111 Course assessment • Course work 40%: i. iii. 20% Mid Term Exams 10% Laboratory work 10% Quiz • Final Exam 60% Total: 100% 4
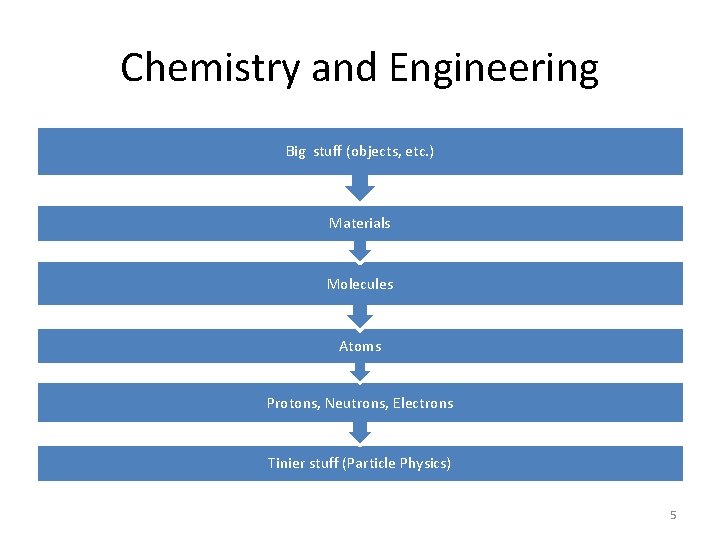
Chemistry and Engineering Big stuff (objects, etc. ) Materials Molecules Atoms Protons, Neutrons, Electrons Tinier stuff (Particle Physics) 5

Advice • If you are worried about chemistry, don’t get behind! Keep up with reading, homework etc • If I seem to think you know more than you really do, let me know • Get help if you need it (classmates, TA`s, me. . ) • Focus on understanding, not just on guessing what might be on exams 6

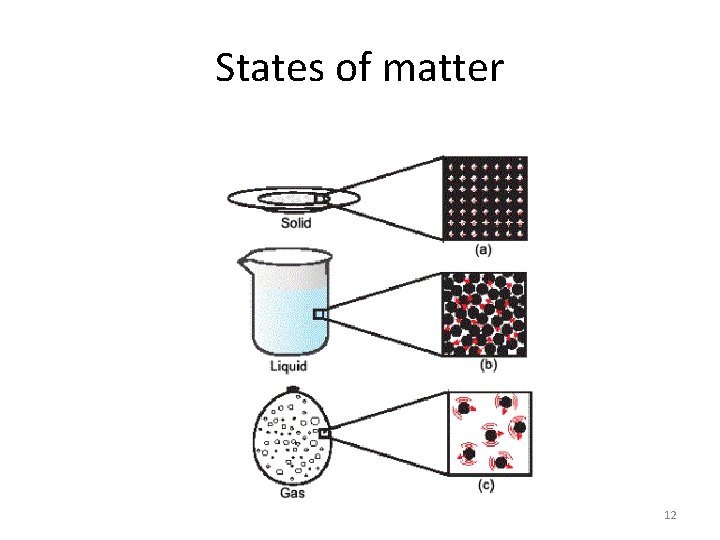
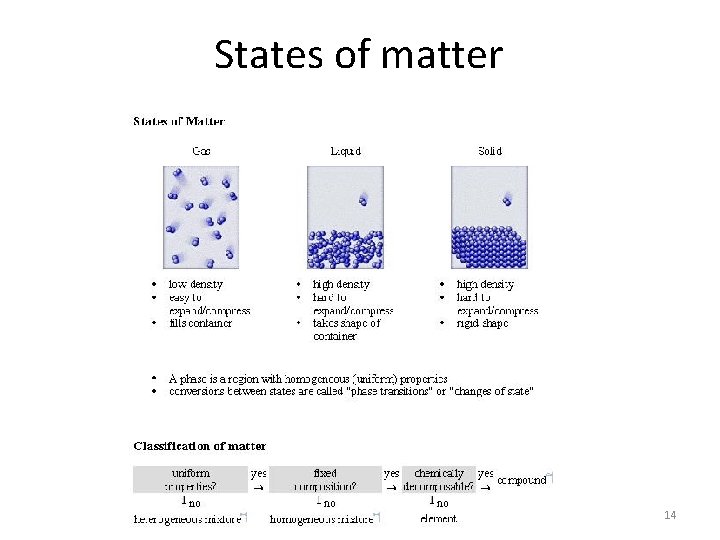
Introduction Look at the nature as a whole can divide things into two main categories: living things non-living things Chemistry plays a vital role in helping us to understand the properties and behavior of all matter. All matter consists of particles and can be divided into three main classes : solids Liquids Gases These are called the three states of matter and are sometimes referred to as phases. They can all be distinguished from one another by the following characteristics. 7

Three states of matter • In nature can divide things into two main categories: living things ( animals, plants, humans) Non-living things ( all the rest) • Chemistry “plays” a vital role in helping us to understand the properties and behavior of all matter • Matter can be divided into: Solids Liquids Gases • Three states (phases) of matter 8

Solids Have a definite shape and offer resistance to any attempt to change that shape. Their volumes are hardly affected by changes in temperature and pressure 9

Liquids Have no definite shape and take the shape of the container. However, they have definite surfaces which limit the amount of the space they occupy. Liquids can flow, meaning that they can change their shape under the influence of very small forces. Like solids, their volumes are slighltly affected by changes in temperature and pressure. 10

Gases They are like liquids in that they have no definite shape and can flow. Unlike a liquid a gas has no bounding surface and will fill completely any container in which it is placed. The volume of gases are very much affected by even small changes in temperature and pressure. 11
States of matter 12

Three states of water 13
States of matter 14
Particles 15

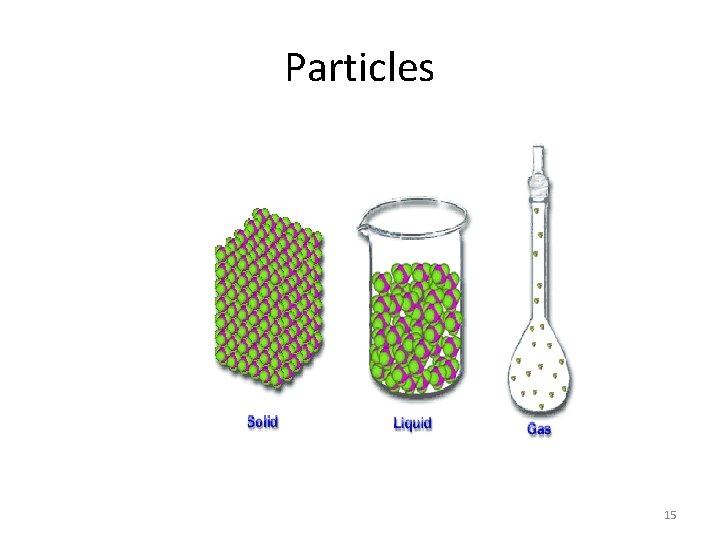
Solid state • Particles of solids are held in place by strong electrostatic forces and are densely packed together. Particles of solids vibrate constantly due to their internal energy but they cannot move from one place to another. Particles of solids possess only vibrational energy. 16

LIQUID STATE • Particles of liquids are kept together by forces of attraction that are weaker than those of solid particles. Within the walls of the container they can move from place to place bumping into the sides of the container and into other particles. This type of energy is called translational energy. This energy gives a liquid the ability to flow and be poured and to spread when a liquid is spilled. Liquid particles also have vibrational energy. 17

GAS STATE • Particles of gases are "more rarefied" than either liquids or solids. This means that the forces of attraction that hold them together are very weak and that the spaces between them are much larger than the spaces between solid and liquid particles. Particles of gases can move from place to place within a container bumping against the walls of the container and against other particles. They rotate and vibrate at the same time. Particles of gases have rotational, translational and vibrational energy. This explains why they can escape from a container very easily. They can put pressure on the side of the container (example a balloon or a tire). 18
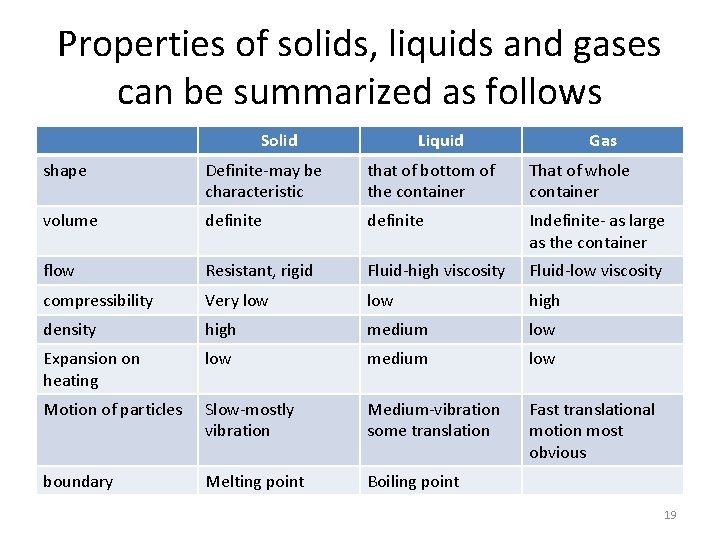
Properties of solids, liquids and gases can be summarized as follows Solid Liquid Gas shape Definite-may be characteristic that of bottom of the container That of whole container volume definite Indefinite- as large as the container flow Resistant, rigid Fluid-high viscosity Fluid-low viscosity compressibility Very low high density high medium low Expansion on heating low medium low Motion of particles Slow-mostly vibration Medium-vibration some translation Fast translational motion most obvious boundary Melting point Boiling point 19
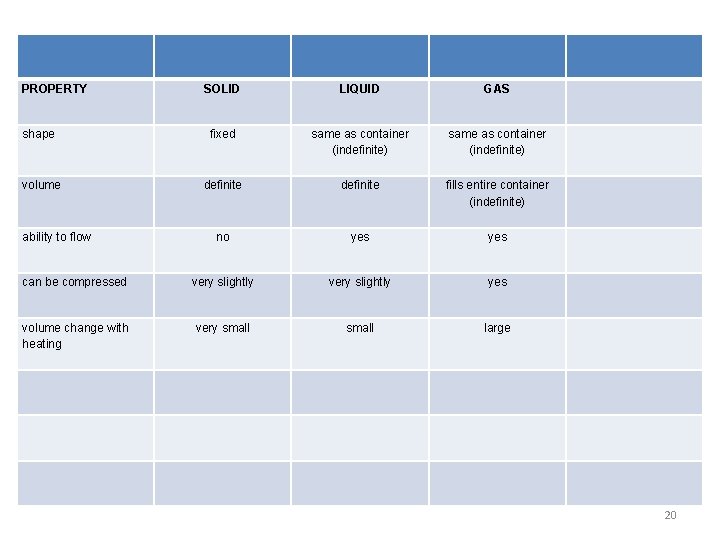
PROPERTY SOLID LIQUID GAS shape fixed same as container (indefinite) volume definite fills entire container (indefinite) no yes can be compressed very slightly yes volume change with heating very small large ability to flow 20
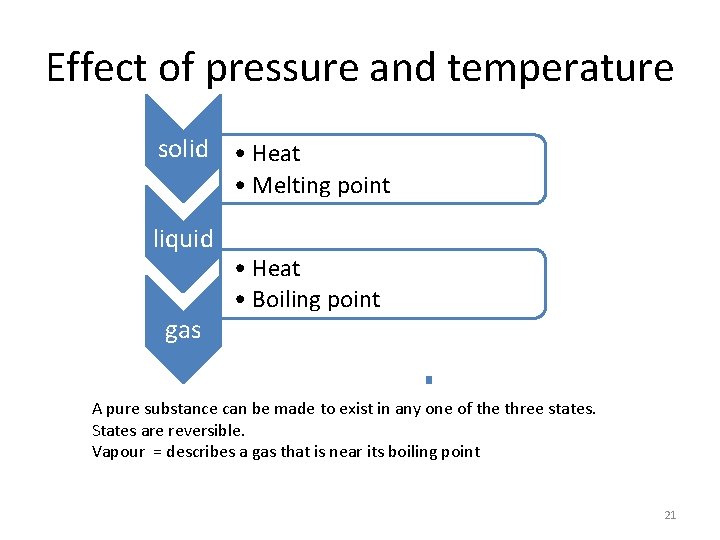
Effect of pressure and temperature solid • Heat • Melting point liquid gas • Heat • Boiling point A pure substance can be made to exist in any one of the three states. States are reversible. Vapour = describes a gas that is near its boiling point 21
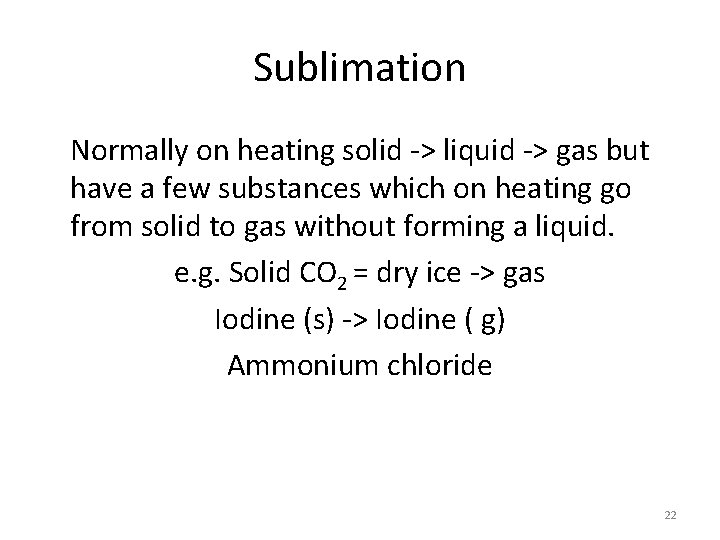
Sublimation Normally on heating solid -> liquid -> gas but have a few substances which on heating go from solid to gas without forming a liquid. e. g. Solid CO 2 = dry ice -> gas Iodine (s) -> Iodine ( g) Ammonium chloride 22
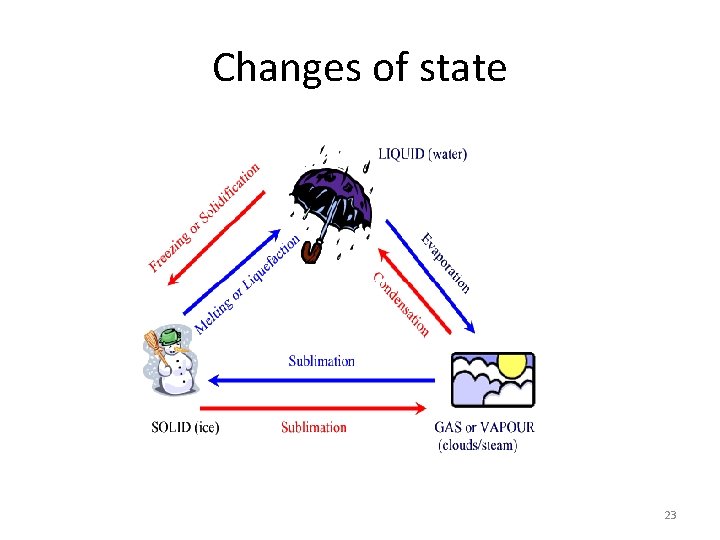
Changes of state 23
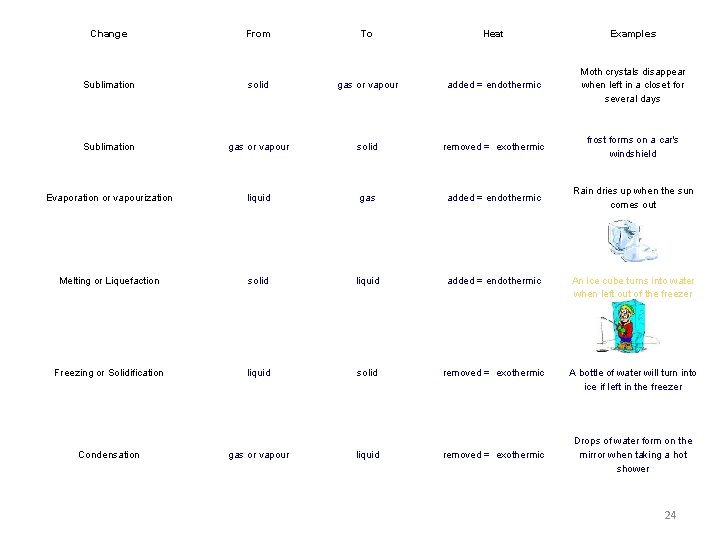
Change From To Heat Examples Sublimation solid gas or vapour added = endothermic Moth crystals disappear when left in a closet for several days Sublimation gas or vapour solid removed = exothermic frost forms on a car's windshield Evaporation or vapourization liquid gas added = endothermic Rain dries up when the sun comes out Melting or Liquefaction solid liquid added = endothermic An ice cube turns into water when left out of the freezer Freezing or Solidification liquid solid removed = exothermic A bottle of water will turn into ice if left in the freezer Condensation gas or vapour liquid removed = exothermic Drops of water form on the mirror when taking a hot shower 24
Types of change Two main types: I. Physical changes II. Chemical changes 25
Physical change • • Produces no new kind of matter It is generally reversible It is not accompanied by great heat change Produces no change of mass ( of the substances) Examples: • All normal changes of state melting, solidification, vaporization, liquefaction (condensation) • Magnetization of iron • Heating of a metal wire by electricity 26

Chemical change Always produces a new kind of matter • • It is generally not easily reversible • It is usually accompanied by considerable heat change • Produces individual substances whose masses are different from those of the original individual substances e. g. if two substances, A and B, react chemically producing substances, C and D the masses of the latter will be different from the masses of A and B examples: • The burning of any substance in air • The rusting of iron • The slaking of lime • Explosion of coal-gas or hydrogen with air 27

How to differentiate between them I. In a physical change the particles remain in the original arrangements or more to arrangements which can easily be changed back to the original. No new combinations of particles are found. When a solid melts or boils the particles move further apart but they keep whatever pattern and composition they had at the start. The original materials are left unchanged. 28

II. In a chemical change the particles undergo internal rearrangement and new combinations are formed with new end often very different properties from the original substance. E. g. Hydrogen burns in oxygen to form water A rearrangement of atoms take place: two hydrogen atoms combine with an oxygen atom to give a molecule of water. Properties of water are very different from properties of oxygen and hydrogen (e. g. melting point, boiling point, effect on metals) 29

Evidence to decide between I and II • Has a new substance been formed? Are the properties very different? • Can the change be reversed easily? • Is the energy change, heat, large or small? 30

Electric heater 31
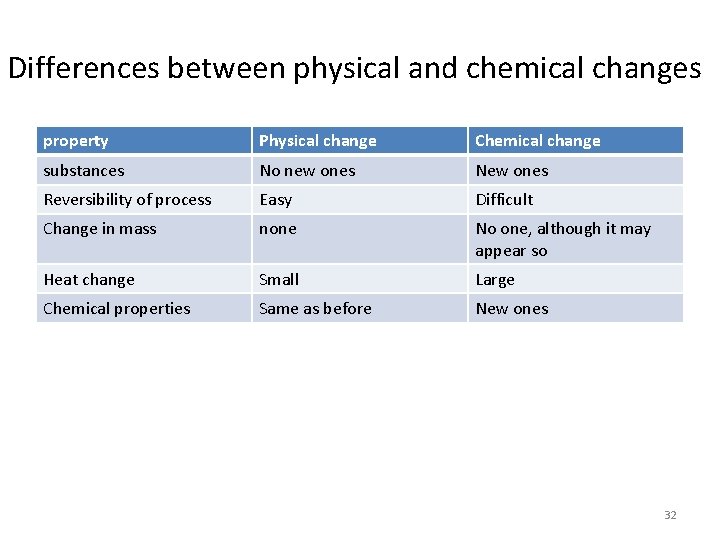
Differences between physical and chemical changes property Physical change Chemical change substances No new ones New ones Reversibility of process Easy Difficult Change in mass none No one, although it may appear so Heat change Small Large Chemical properties Same as before New ones 32

Physical Change No new substance is produced Substance remains the same even with a change of state Definition Properties May require addition of energy Release of energy may occur Outside may look different Inside remains the same Particles may be rearranged Forces of attraction between particles may be weaker Chemical Change substance New substance is always produced Energy is usually released but may be required to get the change going A new substance is produced The particles of the new substance do not resemble those of the old substance Examples Mixing sugar and water Ice melts into water Solid wax ==> Liquid wax Internally, the substance produced is different than the old substances or stronger Final substance is substantially different than initial Vinegar and baking soda mix to form carbon dioxide Hydrochloric acid reacts with magnesium metal to form hydrogen gas 33

Conservation of mass Important principle: Matter can neither be created nor destroyed. In a chemical change there may be an apparent change in mass: a gas may be trapped in the reaction or it may escape. However if all the reagents and all the products are weighed the total mass before the reaction is found to the equal the total mass after the reaction 34
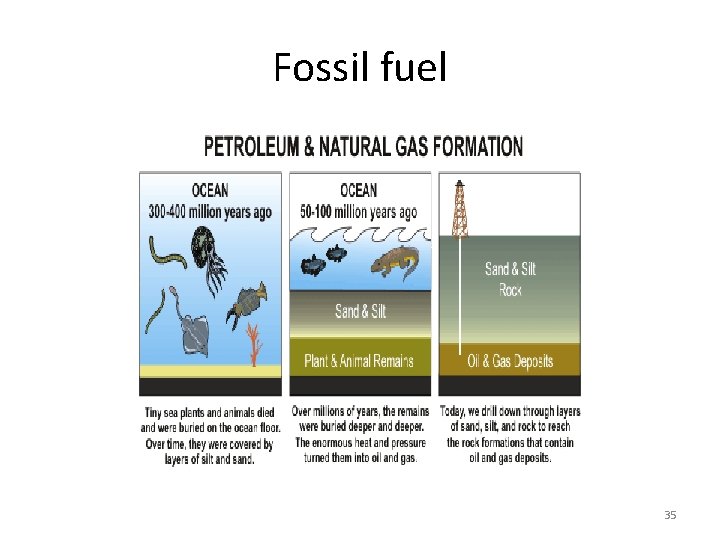
Fossil fuel 35
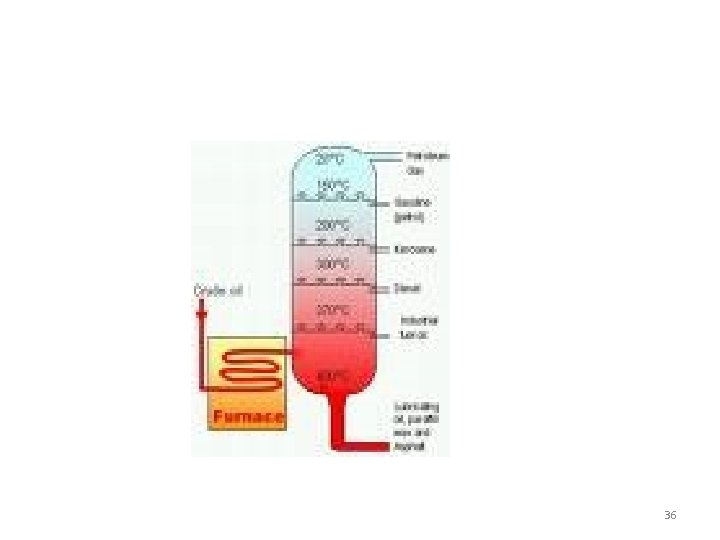
36

Refinery 37
- Slides: 37