Classification of Matter I Composition of Matter Classification




- Slides: 8

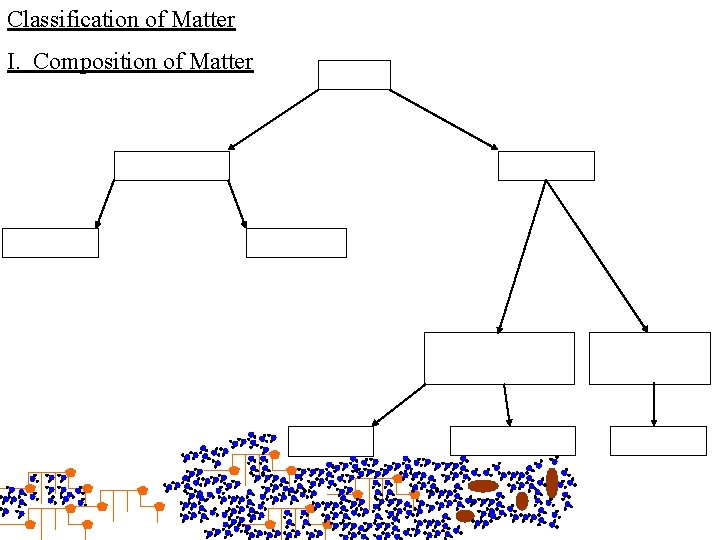
Classification of Matter I. Composition of Matter

Classification of Matter II. Properties of Matter A. Physical Changes -a ______ in ____, _____, or _____ of _____ -_______ of _______ -____ of ______ -_____ of _____ - still has _________ B. Chemical Changes -a ______ in ________ in a ____ to a _________ -_______ of _______

Classification of Matter II. Properties of Matter B. Chemical Changes -____ of _______ 1. Energy -______ is _______ or ____ -____, _____, or ______ 2. Chemical -_____ of ________ -_____ of _______ in _______ -_____ of __________ (______ out of ____) -_______ -___ ______ (____ or ____ in _______ or _______), _______of ____

Classification of Matter II. Properties of Matter B. Chemical Changes 1. Hypothesis: 2. Prediction: 3. Gather Data: A. Safety:

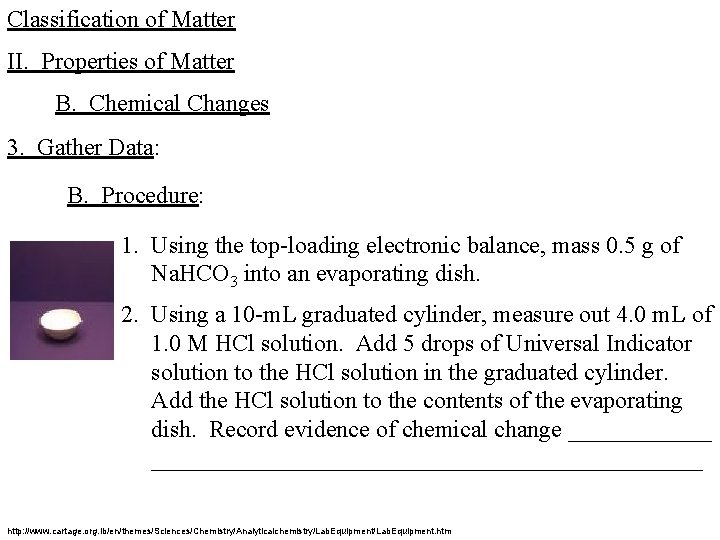
Classification of Matter II. Properties of Matter B. Chemical Changes 3. Gather Data: B. Procedure: 1. Using the top-loading electronic balance, mass 0. 5 g of Na. HCO 3 into an evaporating dish. 2. Using a 10 -m. L graduated cylinder, measure out 4. 0 m. L of 1. 0 M HCl solution. Add 5 drops of Universal Indicator solution to the HCl solution in the graduated cylinder. Add the HCl solution to the contents of the evaporating dish. Record evidence of chemical change _____________________________ http: //www. cartage. org. lb/en/themes/Sciences/Chemistry/Analyticalchemistry/Lab. Equipment. htm

Classification of Matter II. Properties of Matter B. Chemical Changes 3. Gather Data: B. Procedure: 3. Repeat Step 2, adding a second measure of HCl to the contents of the evaporating dish. Record evidence of chemical change_________________ 4. Heat the contents of the evaporating dish to dryness on the hot plate. 4. Analyze Data: A. In the lab, you massed out 0. 5 g of Na. HCO 3. Is Na. HCO 3 a substance or a mixture? ________________ B. Is Na. HCO 3 an element or compound? __________

Classification of Matter II. Properties of Matter B. Chemical Changes 4. Analyze Data: C. In the lab, you added 4. 0 m. L HCl solution, which is made by dissolving 36. 5 g of HCl into enough water to make one liter. Is the HCl solution a substance or a mixture? _______ D. Is HCl an element or a compound? _______ 5. Draw Conclusions: E. When HCl solution was added to the Na. HCO 3 the first time, did a chemical change take place? ______ How did you know? __________________________

Classification of Matter II. Properties of Matter B. Chemical Changes 5. Draw Conclusions: F. After you added a second 4. 0 m. L HCl solution to your evaporating dish and heated the material in the dish to dryness, a white crystalline solid was left over in the dish. Was this white crystalline solid Na. HCO 3? ______ How do you know? __________________________ G. What do you think the white crystalline solid in the evaporating dish was? ________ H. What do you think the colorless, odorless, gas given off when you added the HCl solution to the Na. HCO 3 was? ______
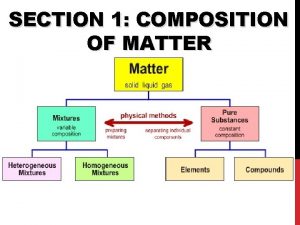
 Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Does copper have uniform composition
Does copper have uniform composition Composition of matter which depends on temperature
Composition of matter which depends on temperature Study of composition structure and properties
Study of composition structure and properties What is dissolution
What is dissolution Uniform composition
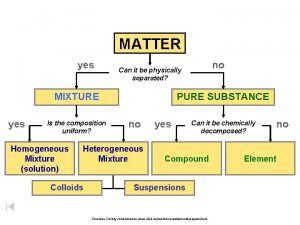
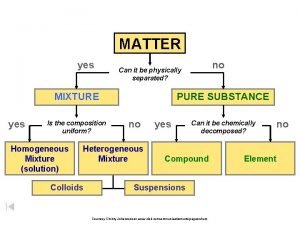
Uniform composition Classifying matter flow chart
Classifying matter flow chart