COMPOSITION OF MATTER NOTES Chapter 2 MATTER Matter


- Slides: 13

COMPOSITION OF MATTER NOTES Chapter 2

MATTER Matter is anything that has mass and takes up space (volume) – comes in 3 states Mass and weight are different! Mass is the quantity of matter in an object while weight is the force of gravity exerted on an object. The greater the mass of an object indicates the greater force of gravity exerted on that object.

PROPERTIES OF MATTER 1. Physical Properties are characteristics of matter than can be observed/measured without permanently changing the identity of the matter. Two most important physical properties: 1. Matter 2. Mass Other physical properties are color, shape, odor, texture, and hardness.

PROPERTIES OF MATTER Chemical properties describe a substances ability to change into a completely new substance. It is almost impossible to reverse a chemical change

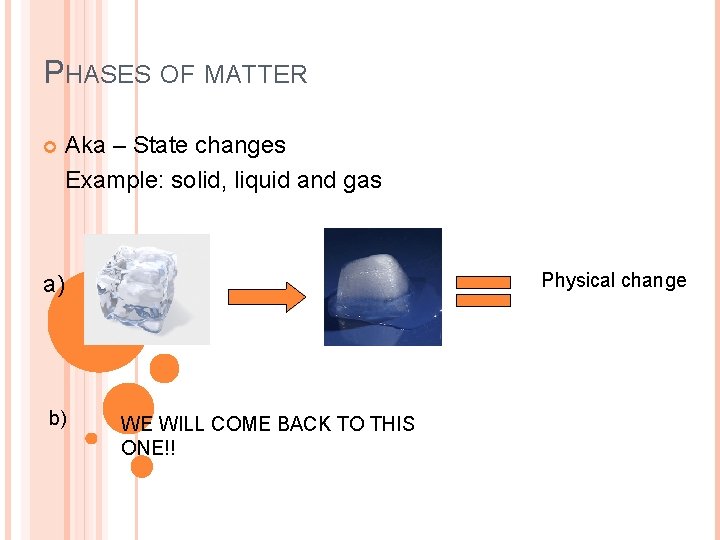
PHASES OF MATTER Aka – State changes Example: solid, liquid and gas Physical change a) b) WE WILL COME BACK TO THIS ONE!!

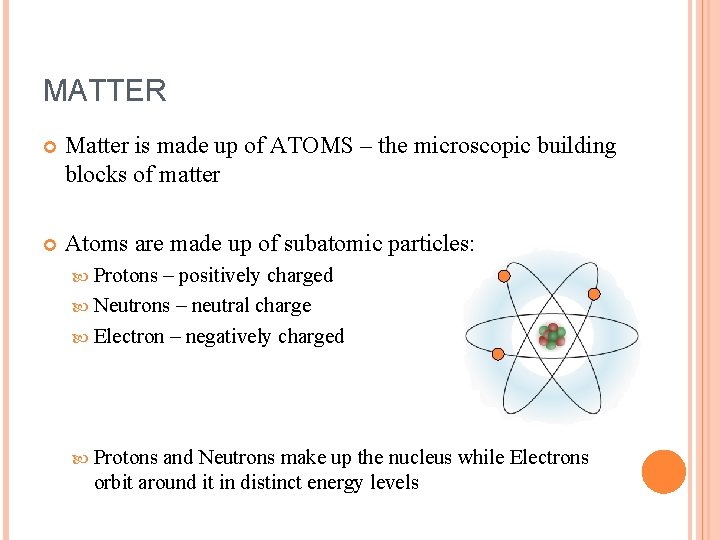
MATTER Matter is made up of ATOMS – the microscopic building blocks of matter Atoms are made up of subatomic particles: Protons – positively charged Neutrons – neutral charge Electron – negatively charged Protons and Neutrons make up the nucleus while Electrons orbit around it in distinct energy levels

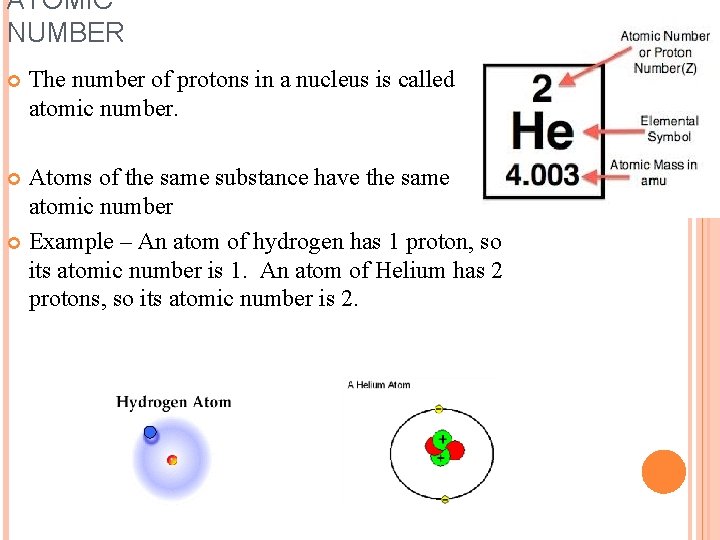
ATOMIC NUMBER The number of protons in a nucleus is called the atomic number. Atoms of the same substance have the same atomic number Example – An atom of hydrogen has 1 proton, so its atomic number is 1. An atom of Helium has 2 protons, so its atomic number is 2.

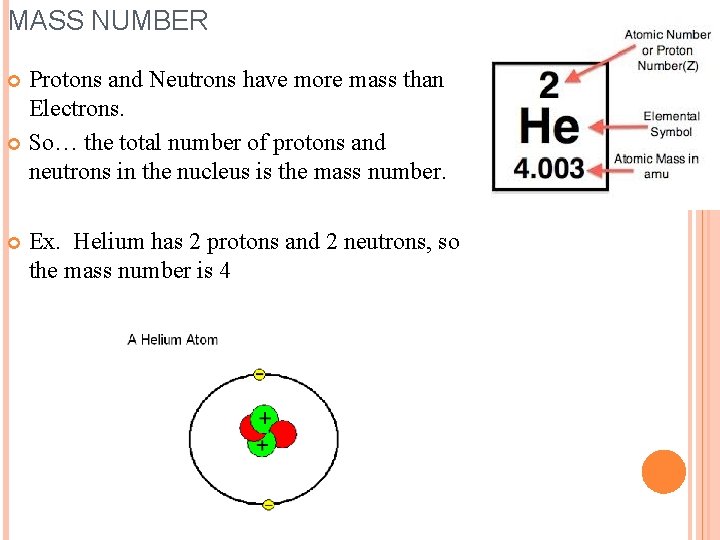
MASS NUMBER Protons and Neutrons have more mass than Electrons. So… the total number of protons and neutrons in the nucleus is the mass number. Ex. Helium has 2 protons and 2 neutrons, so the mass number is 4

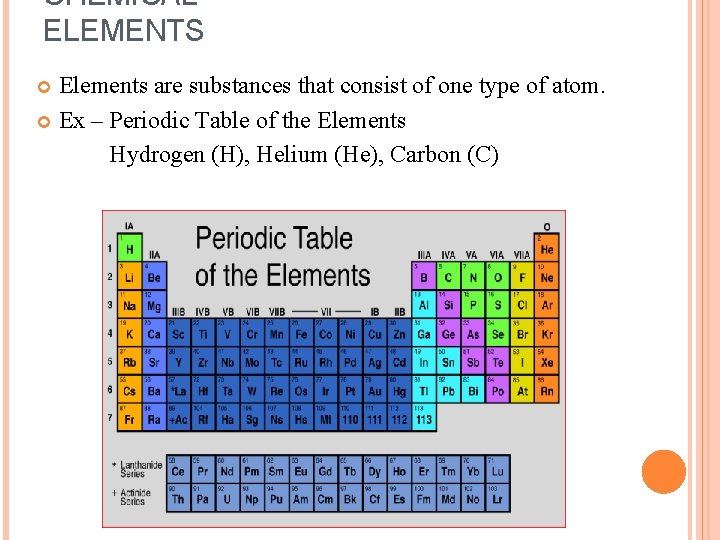
CHEMICAL ELEMENTS Elements are substances that consist of one type of atom. Ex – Periodic Table of the Elements Hydrogen (H), Helium (He), Carbon (C)

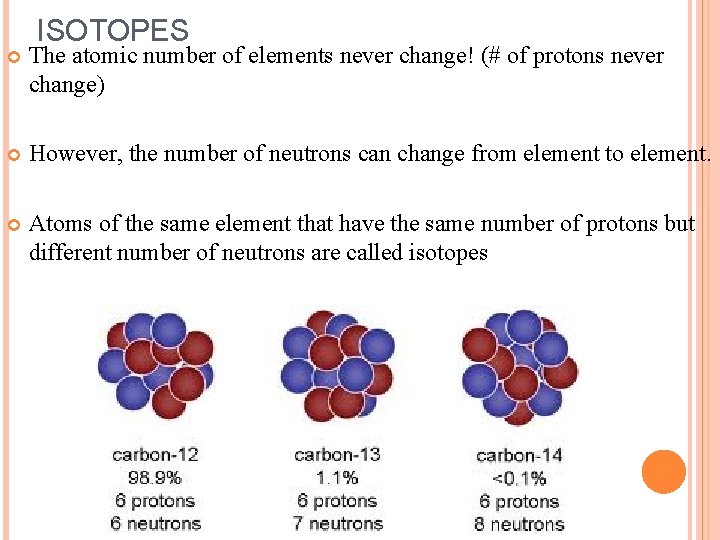
ISOTOPES The atomic number of elements never change! (# of protons never change) However, the number of neutrons can change from element to element. Atoms of the same element that have the same number of protons but different number of neutrons are called isotopes

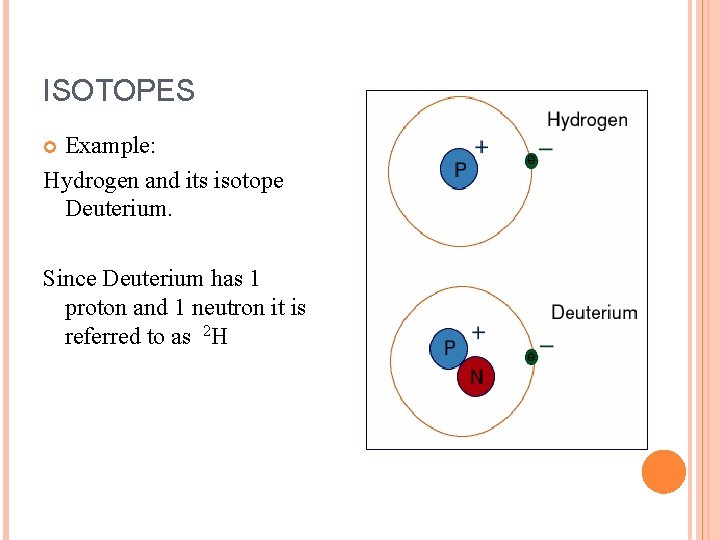
ISOTOPES Example: Hydrogen and its isotope Deuterium. Since Deuterium has 1 proton and 1 neutron it is referred to as 2 H

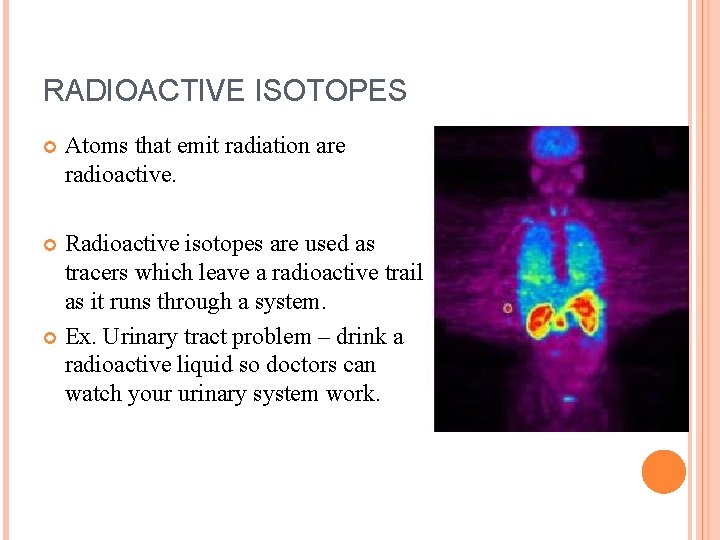
RADIOACTIVE ISOTOPES Atoms that emit radiation are radioactive. Radioactive isotopes are used as tracers which leave a radioactive trail as it runs through a system. Ex. Urinary tract problem – drink a radioactive liquid so doctors can watch your urinary system work.

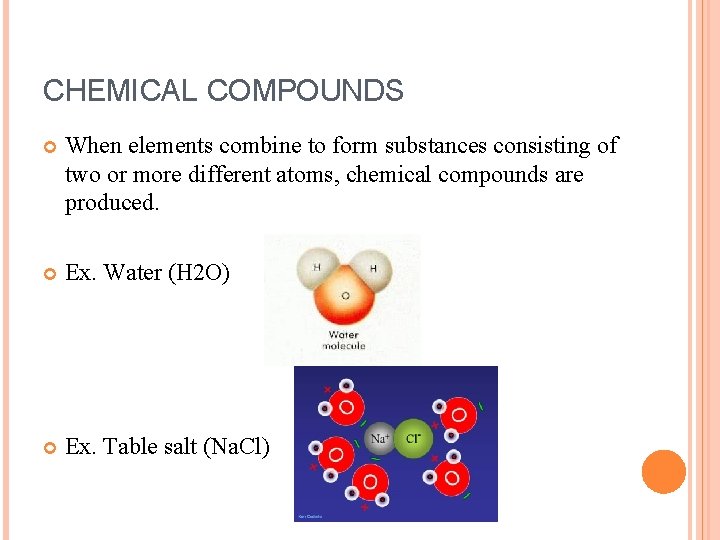
CHEMICAL COMPOUNDS When elements combine to form substances consisting of two or more different atoms, chemical compounds are produced. Ex. Water (H 2 O) Ex. Table salt (Na. Cl)