Matter II Classification of Matter w Matter Flowchart









- Slides: 12

Matter II. Classification of Matter w Matter Flowchart w Pure Substances w Mixtures

A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

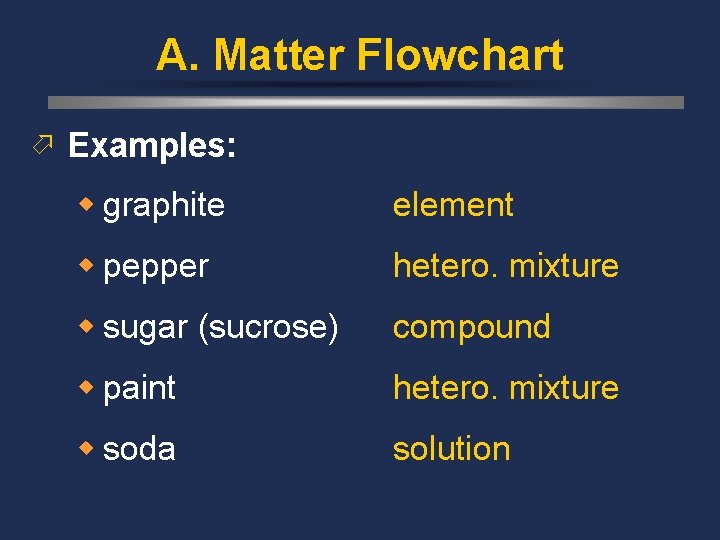
A. Matter Flowchart ö Examples: w graphite element w pepper hetero. mixture w sugar (sucrose) compound w paint hetero. mixture w soda solution

B. Pure Substances ö Element w composed of identical atoms w EX: copper wire, aluminum foil

B. Pure Substances ö Compound w composed of 2 or more elements in a fixed ratio w properties differ from those of individual elements w EX: table salt (Na. Cl)

B. Pure Substances ö Law of Definite Composition w A given compound always contains the same, fixed ratio of elements. ö Law of Multiple Proportions w Elements can combine in different ratios to form different compounds.

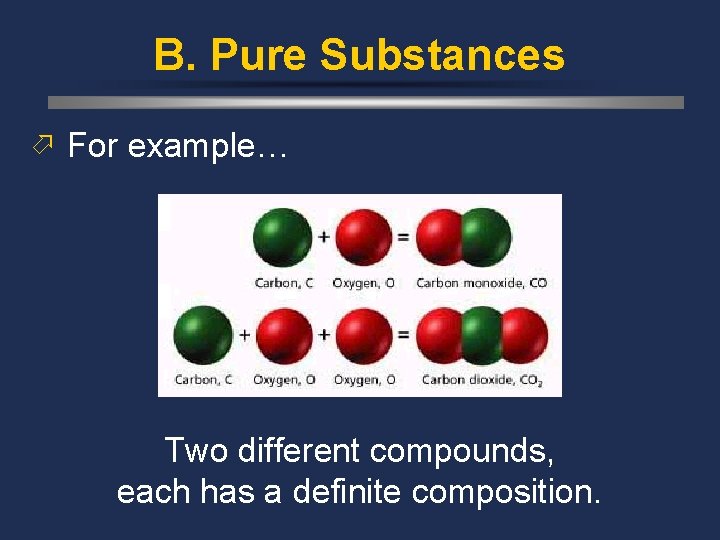
B. Pure Substances ö For example… Two different compounds, each has a definite composition.

C. Mixtures ö Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

C. Mixtures ö Solution w Homogeneous (same) w very small particles w no Tyndall effect w particles don’t settle w EX: rubbing alcohol Tyndall Effect

C. Mixtures ö Colloid w heterogeneous w medium-sized particles w Tyndall effect w particles don’t settle w EX: milk

C. Mixtures ö Suspension w heterogeneous w large particles w Tyndall effect w particles settle w EX: fresh-squeezed lemonade

C. Mixtures ö Examples: w mayonnaise colloid w muddy water suspension w fog colloid w saltwater solution w Italian salad dressing suspension
 Classification of matter flowchart
Classification of matter flowchart Flowchart for matter
Flowchart for matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Flowchart of matter
Flowchart of matter Matter flowchart chemistry
Matter flowchart chemistry Homogeneous mixture
Homogeneous mixture Flowchart of matter
Flowchart of matter Flowchart of matter
Flowchart of matter Classification of matter class 9
Classification of matter class 9 Classes of lipids
Classes of lipids Classification of joints
Classification of joints