MATTER All matter is made up of very









- Slides: 11

MATTER • All matter is made up of very small particles. • All particles in a pure substance are the same. • There is space between the particles. • The particles are always moving. • The particles in a substance are attracted to each other. The strength of the attraction depends upon the type of particle.

MATTER All matter exists in one of three states: • Solid – has a definite shape and volume (e. g. Sugar) • • Liquid – has a definite volume but no definite shape (e. g. Water) • • Gas – no definite shape nor a definite volume (e. g. Oxygen)


Solid - Particles in a fixed order and location - Particles can only move in their fixed location - Cannot be compressed - Cannot flow

Liquids - Particles not in a fixed order or location - Particles move freely, but slowly - Cannot be compressed easily - Can Flow - Takes the shape of the container it is pour into

Gases - Particles not in any organized pattern - Particles move freely and very quickly - Can be compressed - Takes the shape of the entire container

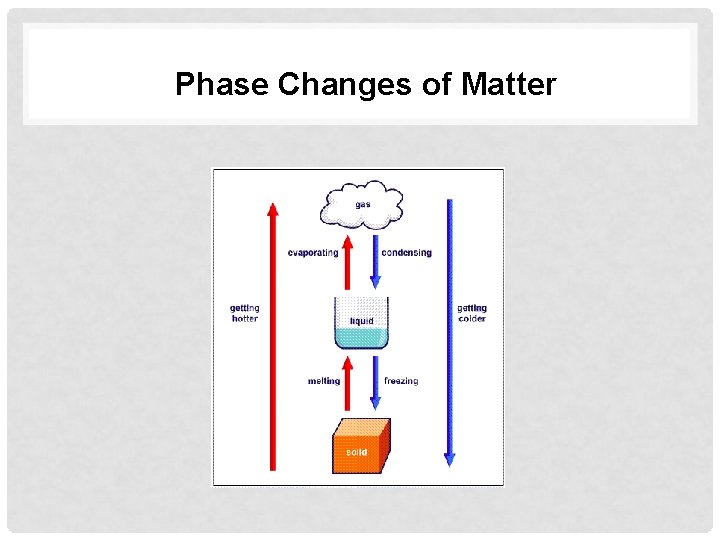
Phase Changes of Matter

Temperature and Theory of Kinetic Energy - Kinetic Energy – Energy produced by the constant motion and collision of particles of matter - - Temperature – The measure of the amount of kinetic energy in matter How are Kinetic Energy and Temperature related?

Heat and Particle Theory - Heat of fusion – The amount of energy required to turn a sample of solid matter into a liquid (ex. Ice to water) - Heat of Vaporization – The amount of energy required to turn a sample of liquid matter into a gas

DENSITY OF SOLIDS, LIQUIDS & GASES • Does water as a liquid, water a solid, or water as a gas have the highest density? Explain.

DENSITY • Density can be described as the “crowdedness” of the particles that make up matter. • When you describe something as being “heavy” or “light” you are referring to that something’s density. • Each substance has its own, unique density. • The closer the particles are together in a substance, the higher its density is.
 All matter is made up of
All matter is made up of Element mixture and compound diagram
Element mixture and compound diagram All matter is made up of
All matter is made up of Very bad to very good scale
Very bad to very good scale Multiplication of scientific notation
Multiplication of scientific notation Very little or very few
Very little or very few Is a very shallow skillet with very short sloping sides
Is a very shallow skillet with very short sloping sides Quantifiers of food
Quantifiers of food Name three lines
Name three lines Draino chemical name
Draino chemical name White matter
White matter Matter is made up of
Matter is made up of