Thermal Energy Heat Chapter 14 14 1 Temperature













- Slides: 24

Thermal Energy & Heat Chapter 14

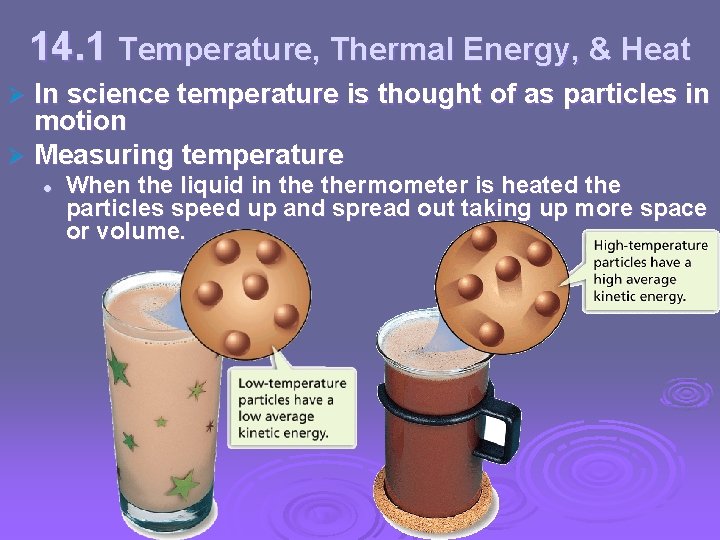
14. 1 Temperature, Thermal Energy, & Heat In science temperature is thought of as particles in motion Ø Measuring temperature Ø l When the liquid in thermometer is heated the particles speed up and spread out taking up more space or volume.

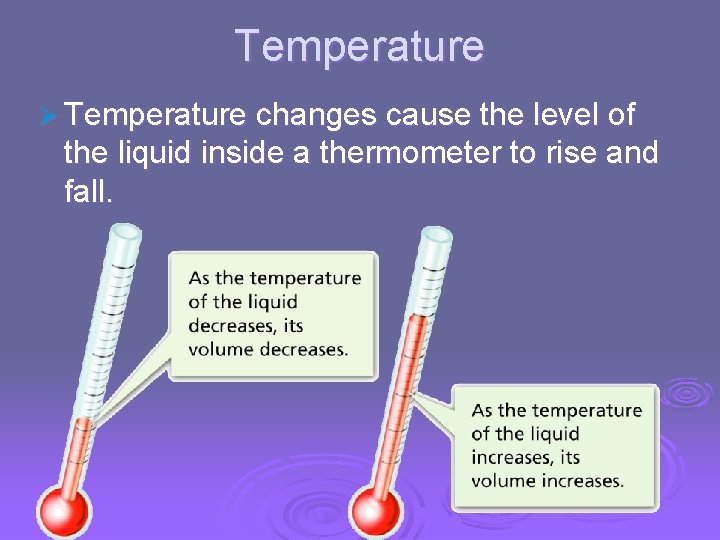
Temperature, Thermal Energy and Heat Temperature Ø Temperature changes cause the level of the liquid inside a thermometer to rise and fall.

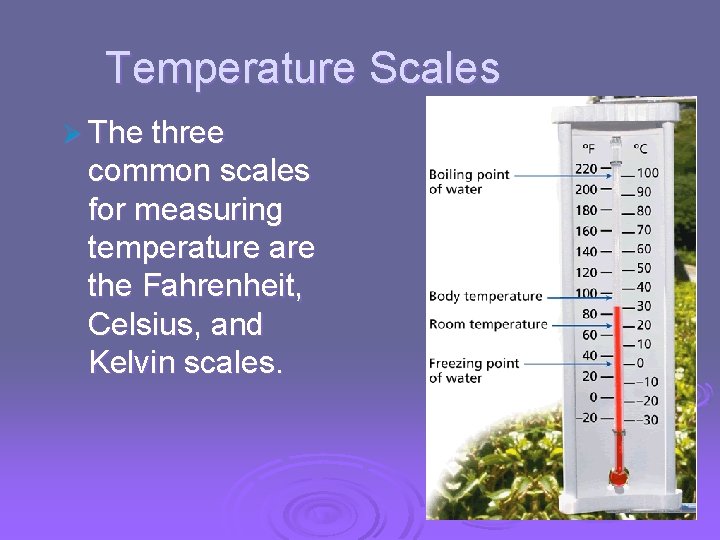
Ø Temperature Scales -Fahrenheit Scale - 32º freezing / 212º boiling -Celsius Scale - 0º freezing / 100º boiling -Kelvin Scale – 273 freezing / 373 boiling -commonly used in physical science -zero on the Kelvin scale is called absolute zero, no more thermal energy can be removed

Temperature, Thermal Energy and Heat Temperature Scales Ø The three common scales for measuring temperature are the Fahrenheit, Celsius, and Kelvin scales.

Ø Thermal Energy l l l Ø depends on the number of particles in the object depends on the temperature of the object depends on the arrangement of the object’s particles Heat l l l In science, objects contain thermal energy, not heat Heat is a transfer of thermal energy, moving from a warmer object to a cooler object The amount of heat required to raise the temperature of an object depends on its chemical makeup this is called specific heat A material with a high specific heat can absorb a great deal of thermal energy without a great change in temperature The energy gained or lost by a specific material is related to its • • • mass change in temp. and specific heat CHANGE IN ENERGY = MASS X SPECIFIC HEAT X CHANGE IN TEMP.

Temperature, Thermal Energy and Heat Converting Units To convert a Fahrenheit temperature to a Celsius temperature, use the following formula: Ø ºC = 5/9 (ºF – 32) Ø For example, if the temperature in your classroom is 68ºF, what is the temperature in degrees Celsius? Ø ºC = 5/9 (68 – 32) Ø ºC = 5/9 X 36 Ø ºC = 20 Ø The temperature of your classroom is 20ºC. Ø

Temperature, Thermal Energy and Heat Converting Units Ø Practice Problem Ø While at the beach, you measure the ocean temperature as 77ºF. What is the temperature of the ocean in degrees Celsius? Ø 25ºC

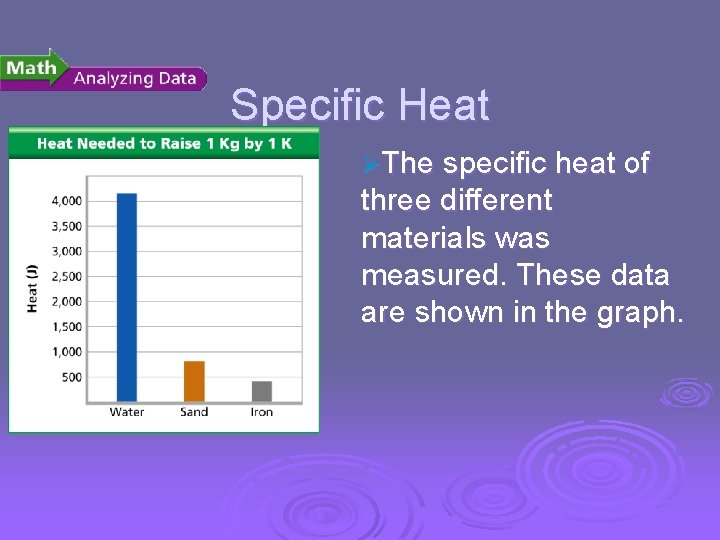
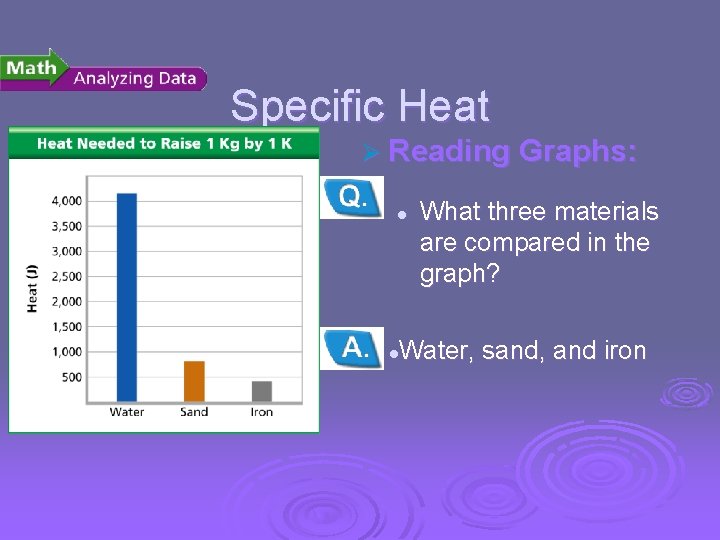
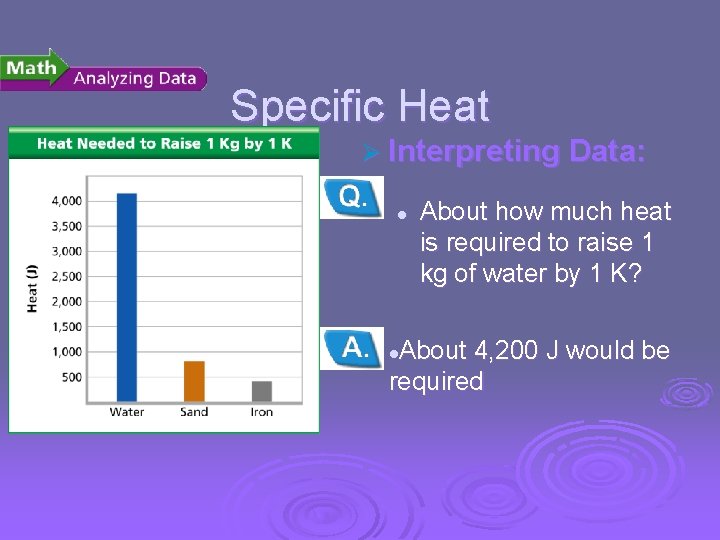
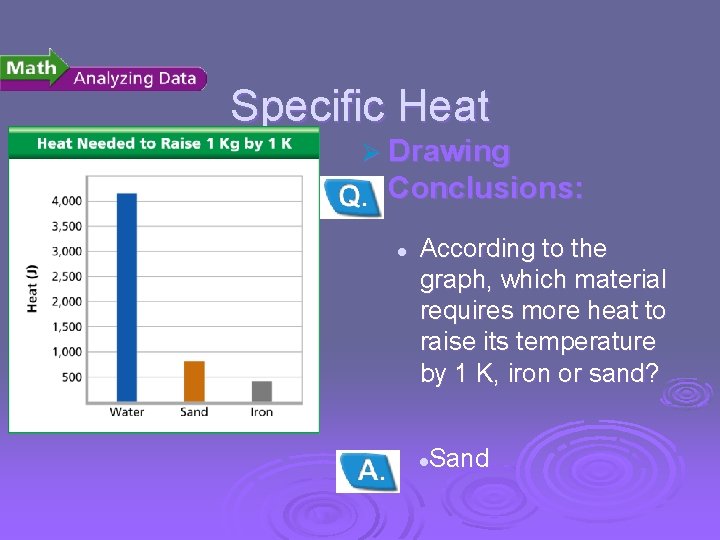
Temperature, Thermal Energy and Heat Specific Heat ØThe specific heat of three different materials was measured. These data are shown in the graph.

Temperature, Thermal Energy and Heat Specific Heat Ø Reading Graphs: l What three materials are compared in the graph? Water, sand, and iron l

Temperature, Thermal Energy and Heat Specific Heat Ø Interpreting Data: l About how much heat is required to raise 1 kg of water by 1 K? About 4, 200 J would be required l

Temperature, Thermal Energy and Heat Specific Heat Ø Drawing Conclusions: l According to the graph, which material requires more heat to raise its temperature by 1 K, iron or sand? Sand l

Temperature, Thermal Energy and Heat Specific Heat Ø A material with a high specific heat can absorb a great deal of thermal energy without a great change in temperature.

Temperature, Thermal Energy and Heat Temperature Ø Click the Video button to watch a movie about temperature.

14. 2 The Transfer of Heat Ø Heat can transfer 3 ways l Conduction • This occurs without the movement of matter • A collision of fast moving particles l Convection • This is a movement of matter (liquids) • Cool, dense particles sink causing warmer, less dense particles to rise l Radiation • Transfer of energy by electromagnetic waves • Does not require matter to transfer thermal energy

Ø Heat moves from a warmer object to a cooler object! Ø Conductors l l Quick to transfer energy Metals Ø Insulators l l Does not conduct heat well Wood, wool, straw, & paper

14. 3 Thermal Energy & States of Matter Ø States of Matter l l (most matter on Earth can exist in three states of matter) Solids • Particles cannot move out of position, only vibrate back and forth l Liquids • Particles are arranged close together, but are free to move l Gases • Particles move so fast that they don’t stay close together

Ø Changes of State l l Requires thermal energy to be absorbed or released Solid-Liquid Changes • Melting • freezing l Liquid-Gas Changes • • • l Evaporation Boiling Condensation Thermal Expansion • As thermal energy of matter increases, its particles spread out & the substance expands

14. 4 Uses of Heat Ø Heat Engines l l Transform thermal energy to mechanical energy Heat engines are classified into 2 categories • External Combustion Engine • Internal Combustion Engine

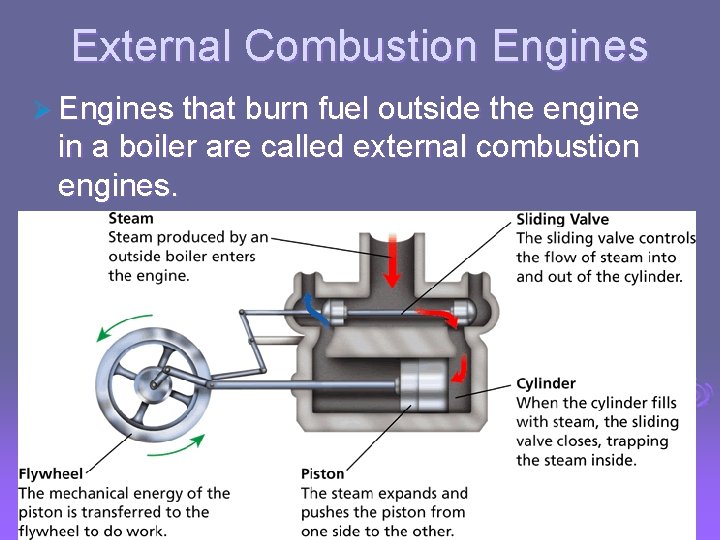
- Uses of Heat External Combustion Engines Ø Engines that burn fuel outside the engine in a boiler are called external combustion engines.

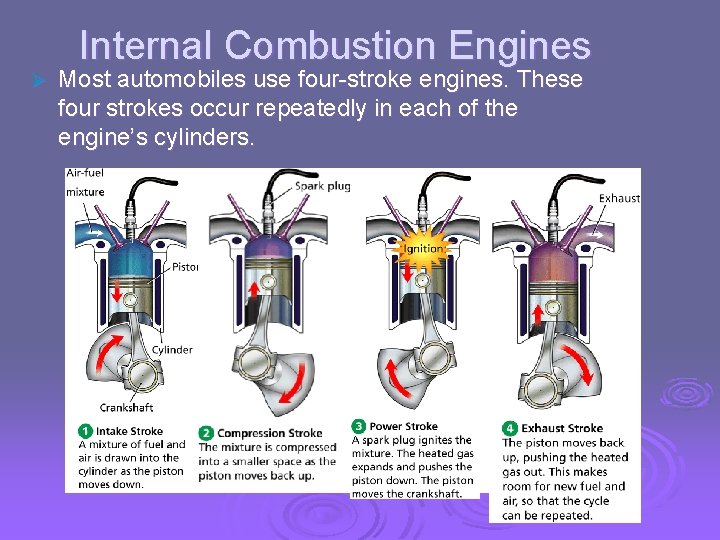
- Uses of Heat Internal Combustion Engines Ø Most automobiles use four-stroke engines. These four strokes occur repeatedly in each of the engine’s cylinders.

Ø Cooling Systems l l Refrigerators Air Conditioners

- Uses of Heat Four-Stroke Engine Activity Ø Click the Active Art button to open a browser window and access Active Art about four-stroke engines.

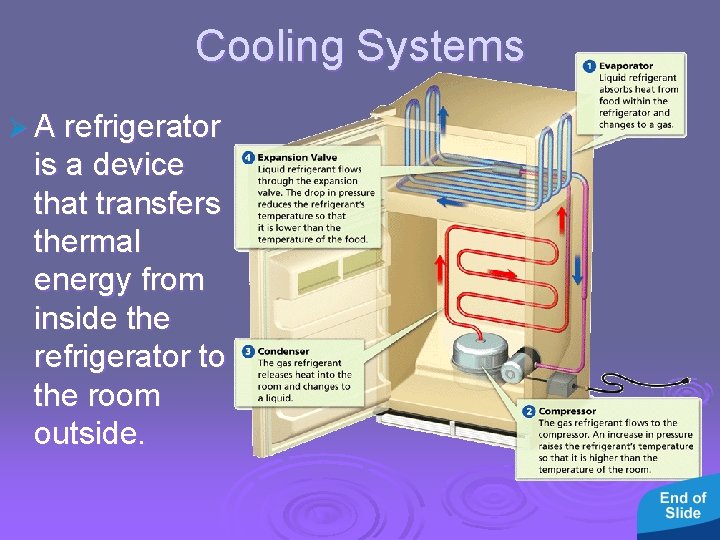
- Uses of Heat Cooling Systems Ø A refrigerator is a device that transfers thermal energy from inside the refrigerator to the room outside.