Thermal Energy and Heat Temperature is the measure









- Slides: 23

Thermal Energy and Heat

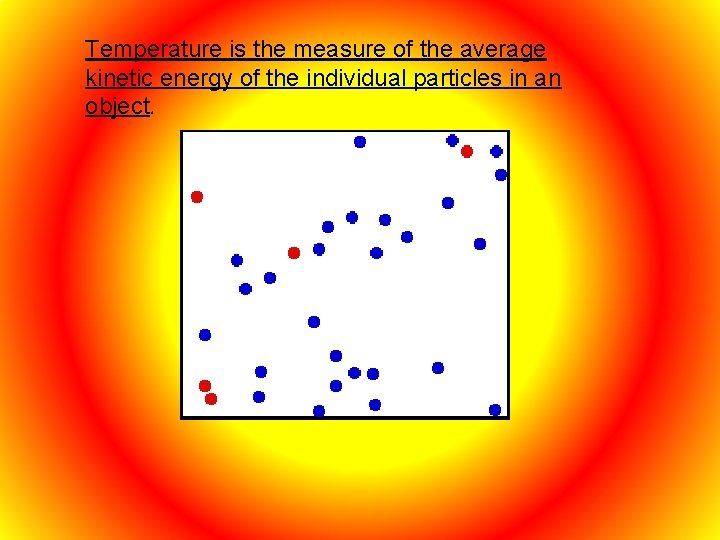
Temperature is the measure of the average kinetic energy of the individual particles in an object.

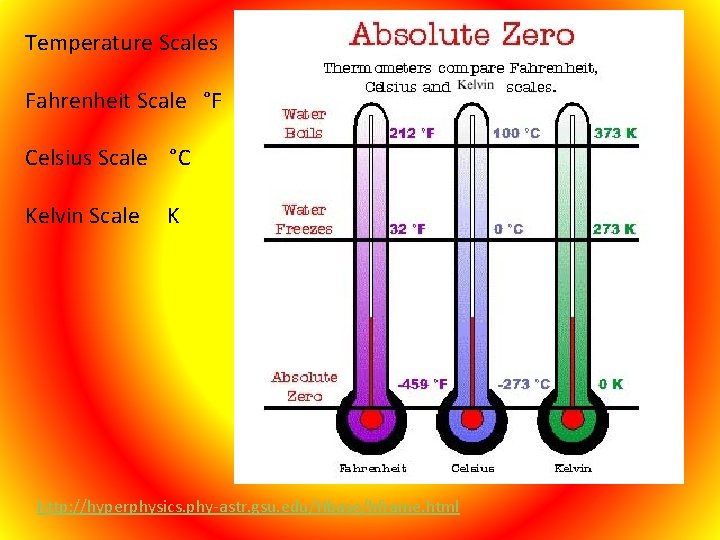
Temperature Scales Fahrenheit Scale °F Celsius Scale °C Kelvin Scale K http: //hyperphysics. phy-astr. gsu. edu/Hbase/hframe. html

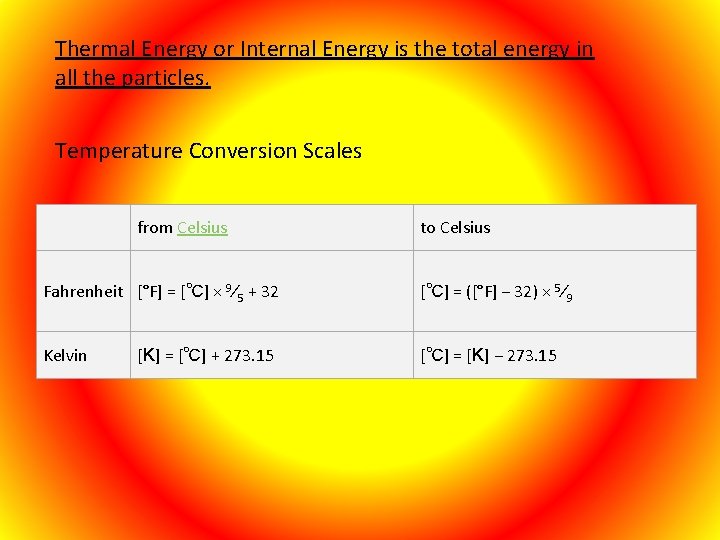
Thermal Energy or Internal Energy is the total energy in all the particles. Temperature Conversion Scales from Celsius to Celsius Fahrenheit [°F] = [℃] × 9⁄5 + 32 [℃] = ([°F] − 32) × 5⁄9 Kelvin [℃] = [K] − 273. 15 [K] = [℃] + 273. 15

The Nature of Heat is the movement of thermal energy from a substance of higher temperature to another at a lower temperature. Heat is thermal energy moving from a warmer object to a cooler object.

Heat is transferred by conduction, convection, and radiation. Conduction is the transfer of energy through matter from particle to particle. It is the transfer and distribution of heat energy from atom to atom within a substance. For example, a spoon in a cup of hot soup becomes warmer because the heat from the soup is conducted along the spoon. Conduction is most effective in solids-but it can happen in fluids. Fun fact: Have you ever noticed that metals tend to feel cold? Believe it or not, they are not colder! They only feel colder because they conduct heat away from your hand. You perceive the heat that is leaving your hand as cold.

Radiation: Electromagnetic waves that directly transport ENERGY through space. Sunlight is a form of radiation that is radiated through space to our planet without the aid of fluids or solids. The energy travels through nothingness! Just think of it! The sun transfers heat through 93 million miles of space. Because there are no solids (like a huge spoon) touching the sun and our planet, conduction is not responsible for bringing heat to Earth. Since there are no fluids (like air and water) in space, convection is not responsible for transferring the heat. Thus, radiation brings heat to our planet. 5770 degrees Kelvin is the surface temperature of the Sun. In the center the temperature is about 15, 000 degrees Celsius.

Convection is the transfer of heat by the actual movement of the warmed matter. Heat leaves the coffee cup as the currents of steam and air rise. Convection is the transfer of heat energy in a gas or liquid by movement of currents. (It can also happen is some solids, like sand. ) The heat moves with the fluid. Consider this: convection is responsible for making macaroni rise and fall in a pot of heated water. The warmer portions of the water are less dense and therefore, they rise. Meanwhile, the cooler portions of the water fall because they are denser.

Heat moves one way from area of high heat to areas of lower heat. Conductors and Insulators Conductors These materials allow heat to flow easily. Insulators don't allow heat to flow easily and we use these materials to stop the flow of heat.

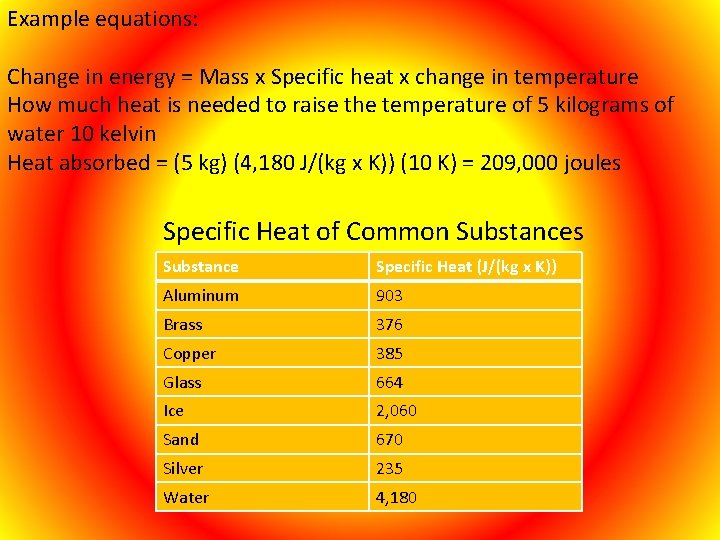
Specific Heat The specific heat is the amount of heat or energy per unit mass (1 Kg) required to raise the temperature by one degree Kelvin. Temperature does not rise at the same rate for all objects. Some objects or material require greater amounts of energy or heat to rise 1 °C. The unit of measure for specific heat is joules per kilogram-kelvin or (J/kg x K)) or Heat Calories Joules per grams-celsius or (J/(g x C)) Or calories per gram. Celsius

Example equations: Change in energy = Mass x Specific heat x change in temperature How much heat is needed to raise the temperature of 5 kilograms of water 10 kelvin Heat absorbed = (5 kg) (4, 180 J/(kg x K)) (10 K) = 209, 000 joules Specific Heat of Common Substances Substance Specific Heat (J/(kg x K)) Aluminum 903 Brass 376 Copper 385 Glass 664 Ice 2, 060 Sand 670 Silver 235 Water 4, 180

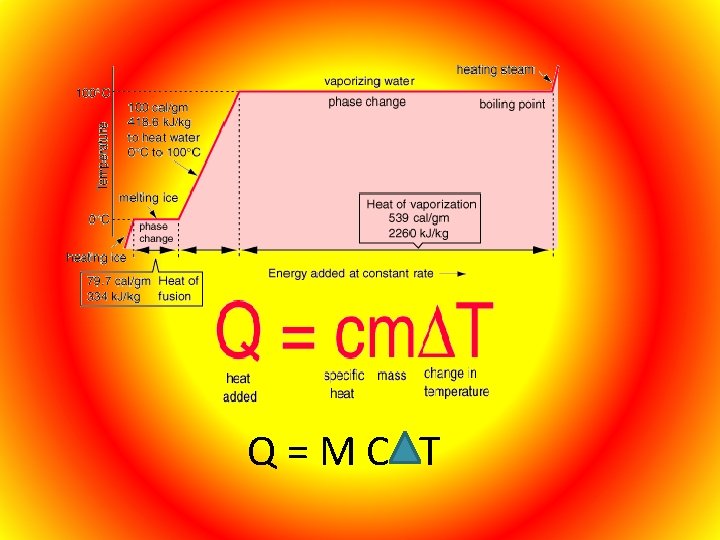
Q = M C T

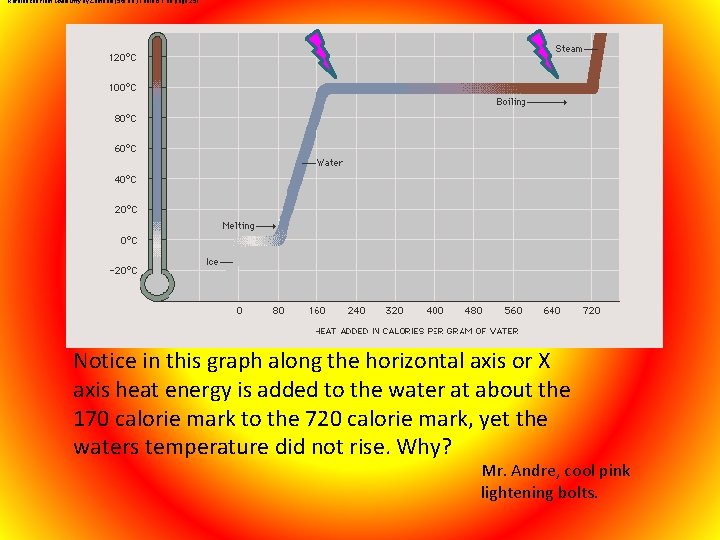
Referenced from Chemistry by Zumdahl (5 th ed. ) Table 6. 1 on page 251 Notice in this graph along the horizontal axis or X axis heat energy is added to the water at about the 170 calorie mark to the 720 calorie mark, yet the waters temperature did not rise. Why? Mr. Andre, cool pink lightening bolts.

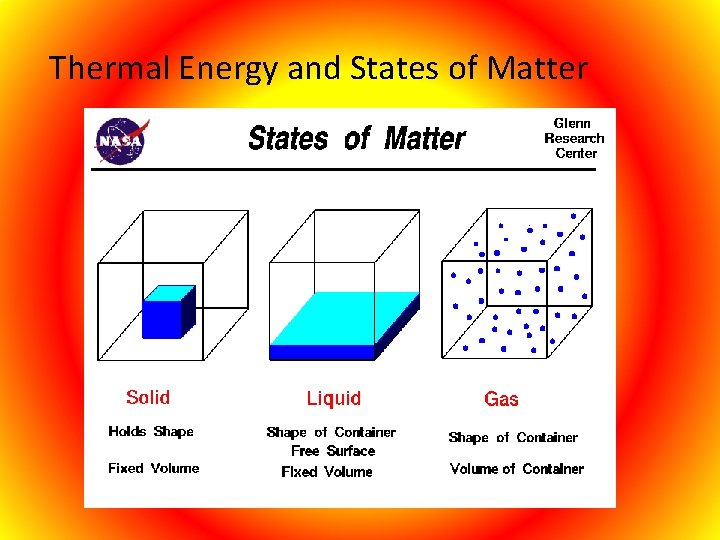
Thermal Energy and States of Matter

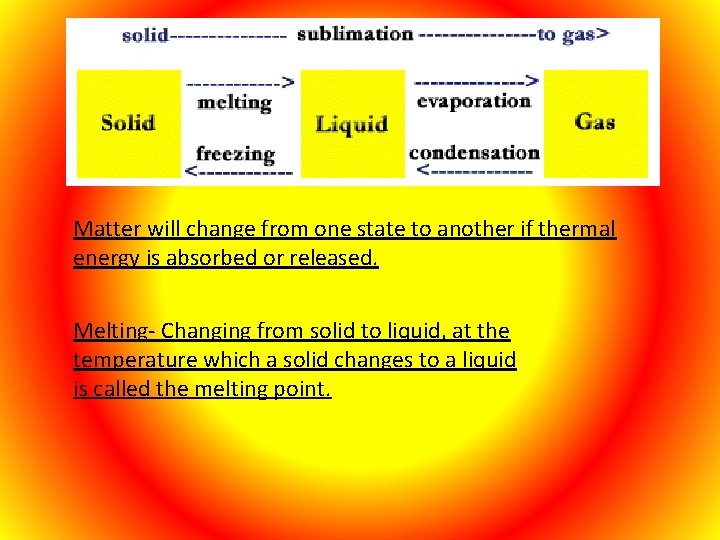
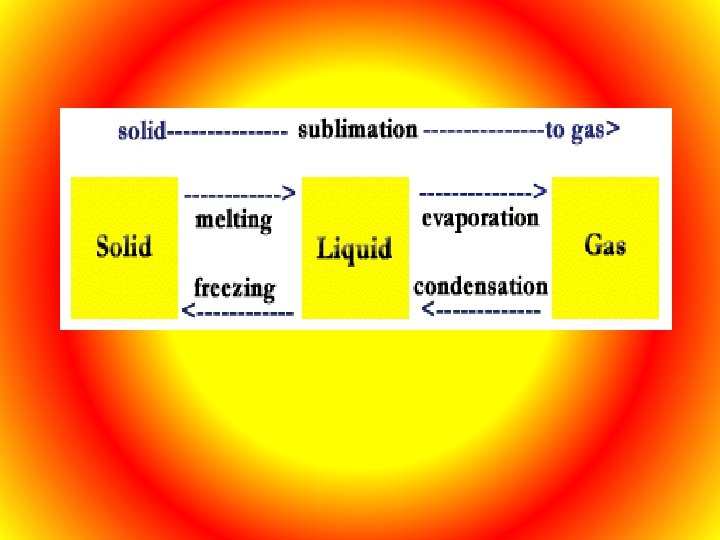
Matter will change from one state to another if thermal energy is absorbed or released. Melting- Changing from solid to liquid, at the temperature which a solid changes to a liquid is called the melting point.

Freezing- Changing from a liquid to a solid, at the temperature when this occurs it is called the freezing point which is the same as its melting point. Vaporization – Process by which matter changes from liquid to gas. Vaporization takes place on the surface of the liquid and is called evaporation. At higher temperatures vaporization can take place below the surface and this is called boiling. Condensation- When water vapor in the air loses its thermal energy when it comes in contact with a solid which is cold. A change from the gas state to the liquid state is condensation.

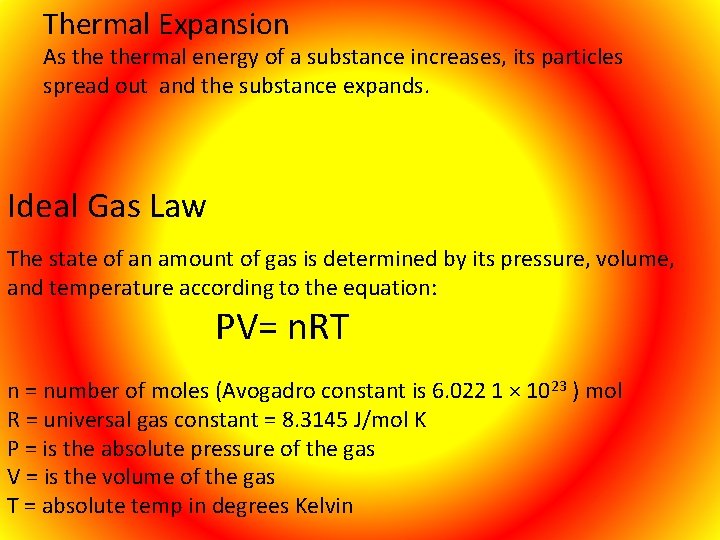
Thermal Expansion As thermal energy of a substance increases, its particles spread out and the substance expands. Ideal Gas Law The state of an amount of gas is determined by its pressure, volume, and temperature according to the equation: PV= n. RT n = number of moles (Avogadro constant is 6. 022 1 × 10 R = universal gas constant = 8. 3145 J/mol K P = is the absolute pressure of the gas V = is the volume of the gas T = absolute temp in degrees Kelvin 23 ) mol

Heat Calculations ΔQ = M C ΔT The specific heat of water is both 4. 18 j/g°c and 1. 0 c/g°c. When ever you use the different specific heat values that will be the units for your heat, either joules or calories. Know, learn it, use it, live it! You are the scientist of tomorrow! Oh No! Quick move to Montana.

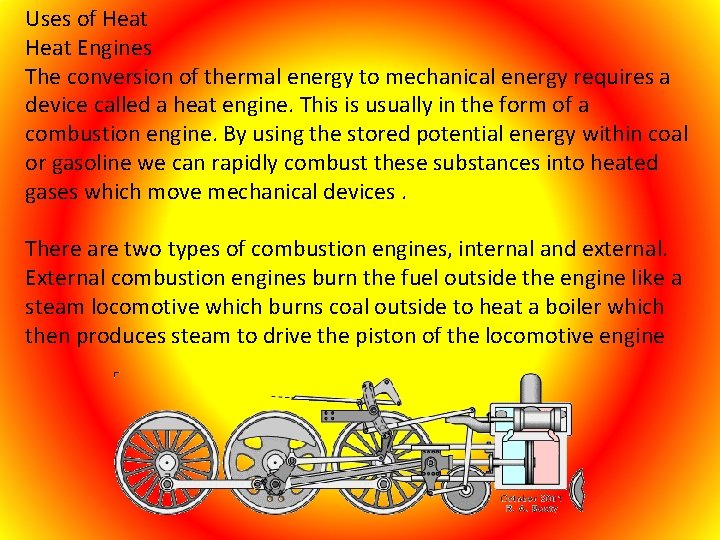
Uses of Heat Engines The conversion of thermal energy to mechanical energy requires a device called a heat engine. This is usually in the form of a combustion engine. By using the stored potential energy within coal or gasoline we can rapidly combust these substances into heated gases which move mechanical devices. There are two types of combustion engines, internal and external. External combustion engines burn the fuel outside the engine like a steam locomotive which burns coal outside to heat a boiler which then produces steam to drive the piston of the locomotive engine

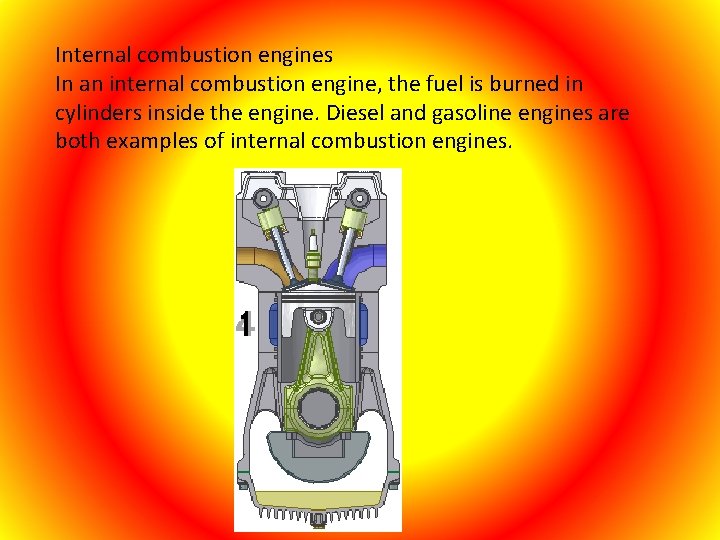
Internal combustion engines In an internal combustion engine, the fuel is burned in cylinders inside the engine. Diesel and gasoline engines are both examples of internal combustion engines.

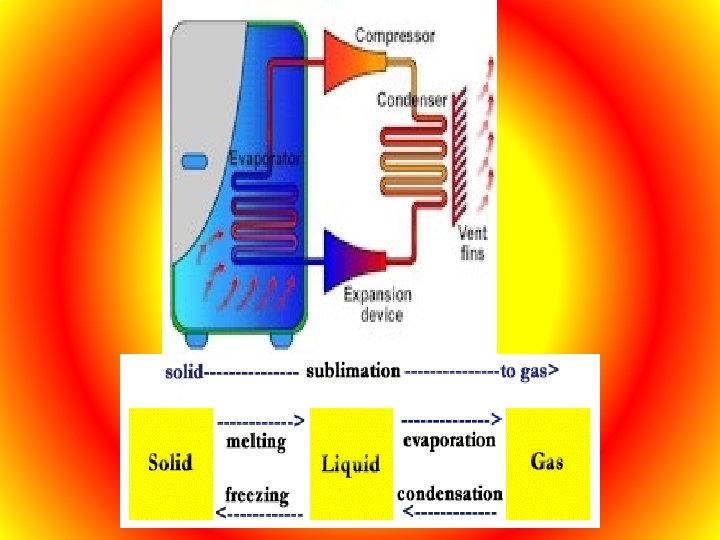
Refrigerators A refrigerator is a device that uses an outside energy source to transfer thermal energy from a cool area to a warm area. The refrigerator motor runs a compressor that compresses the refrigerant (freon) in a gas state which causes its pressure and temperature to rise. When this happens the gas gives off thermal energy. This heat is transferred to the outside air. As the gas loses thermal energy, it changes from gas to liquid. T gas is then allowed to evaporate. As it evaporate it cools. The cold gas is then pumped through tubes in the walls of the refrigerator. There the gas absorbs heat from inside the refrigerator. And so thermal energy is transferred from the space inside the refrigerator to the gas. The gas then returns to the compressor and the next cycle begins

