Energy Table of Contents Temperature Thermal Energy and
Energy Table of Contents Temperature, Thermal Energy, and Heat The Transfer of Heat Thermal Energy and States of Matter Uses of Heat Book M – 6. 1 Pg 176 -181 Book M – 6. 2 Pg 183 -187 Book M – 6. 3 Pg 190 -194 Book M – 6. 4 Pg 195 -199
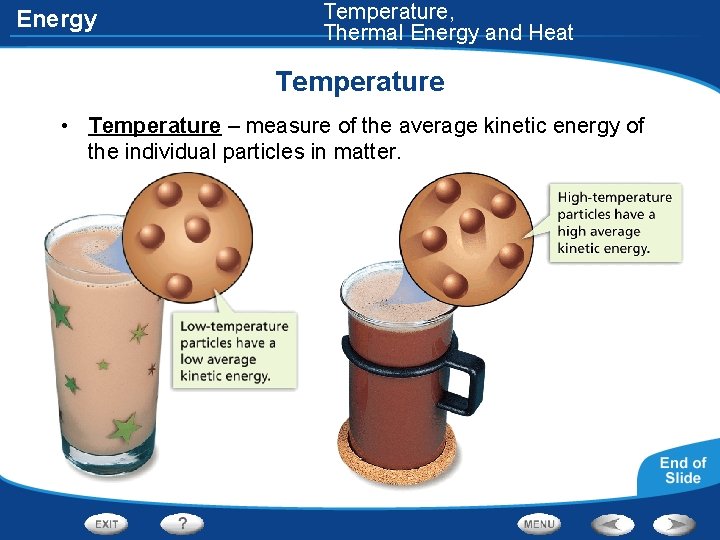
Energy Temperature, Thermal Energy and Heat Temperature • Temperature – measure of the average kinetic energy of the individual particles in matter.
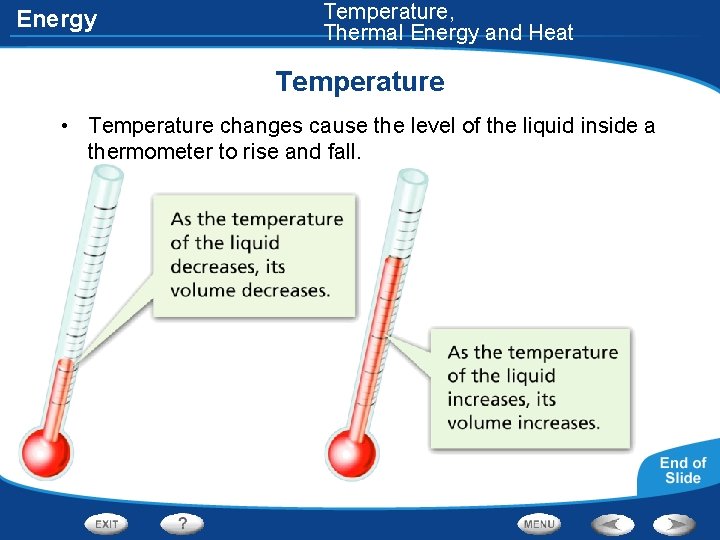
Energy Temperature, Thermal Energy and Heat Temperature • Temperature changes cause the level of the liquid inside a thermometer to rise and fall.
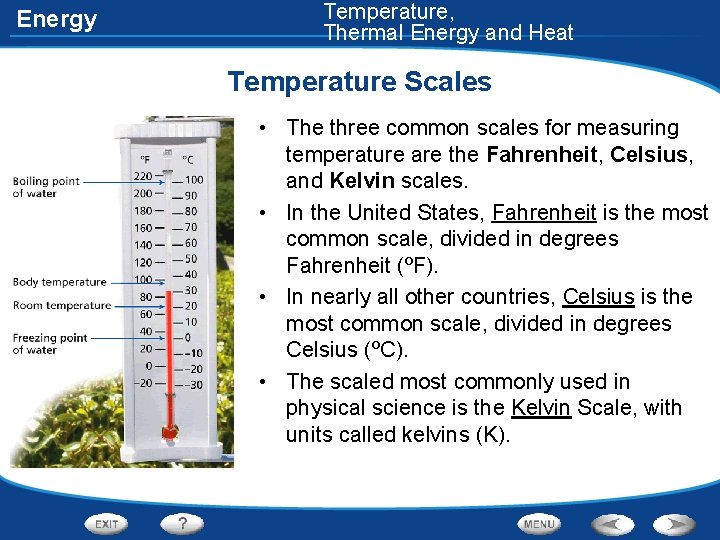
Energy Temperature, Thermal Energy and Heat Temperature Scales • The three common scales for measuring temperature are the Fahrenheit, Celsius, and Kelvin scales. • In the United States, Fahrenheit is the most common scale, divided in degrees Fahrenheit (ºF). • In nearly all other countries, Celsius is the most common scale, divided in degrees Celsius (ºC). • The scaled most commonly used in physical science is the Kelvin Scale, with units called kelvins (K).
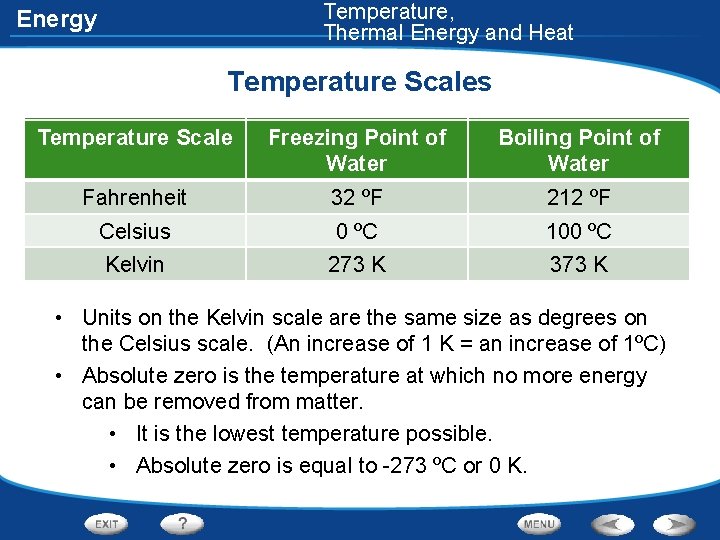
Temperature, Thermal Energy and Heat Energy Temperature Scales Temperature Scale Freezing Point of Water Boiling Point of Water Fahrenheit Celsius 32 ºF 0 ºC 212 ºF 100 ºC Kelvin 273 K 373 K • Units on the Kelvin scale are the same size as degrees on the Celsius scale. (An increase of 1 K = an increase of 1ºC) • Absolute zero is the temperature at which no more energy can be removed from matter. • It is the lowest temperature possible. • Absolute zero is equal to -273 ºC or 0 K.
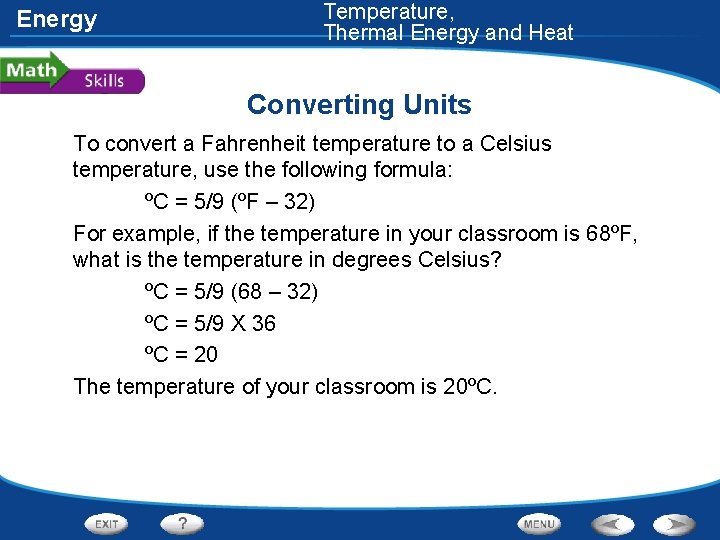
Energy Temperature, Thermal Energy and Heat Converting Units To convert a Fahrenheit temperature to a Celsius temperature, use the following formula: ºC = 5/9 (ºF – 32) For example, if the temperature in your classroom is 68ºF, what is the temperature in degrees Celsius? ºC = 5/9 (68 – 32) ºC = 5/9 X 36 ºC = 20 The temperature of your classroom is 20ºC.

Temperature, Thermal Energy and Heat Energy Converting Units Practice Problem While at the beach, you measure the ocean temperature as 77ºF. What is the temperature of the ocean in degrees Celsius? 25ºC
Energy Thermal Energy and Heat • Temperature, thermal energy, and heat are closely related, but they are all different. • Thermal Energy- the total energy of all of the particles in an object. • Thermal energy depends on the number of particles in the object, temperature of the object, and the arrangement of the object’s particles.

Energy Thermal Energy • The more particles an object has at a given temperature, the more thermal energy it has. o A 1 -liter pot of hot cocoa at 75ºC has more thermal energy than a 0. 2 -liter mug of hot cocoa at 75ºC. o The 1 -liter pot contains more cocoa particles, therefore has more thermal energy. • The higher the temperature of an object, the more thermal energy the object has.
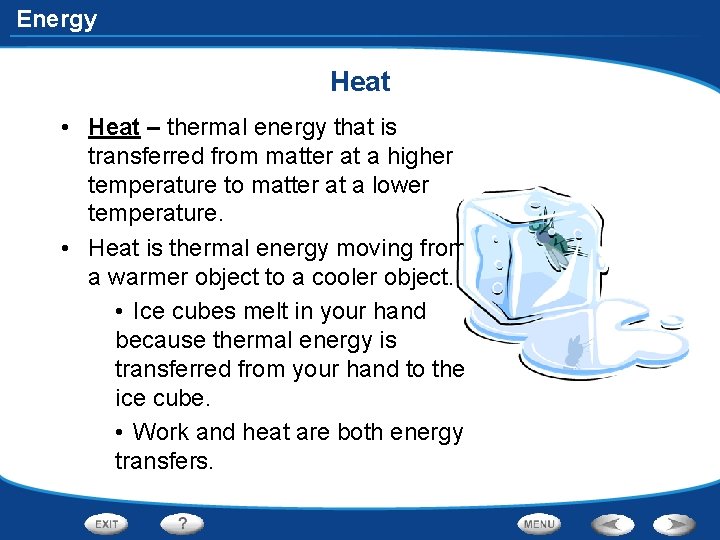
Energy Heat • Heat – thermal energy that is transferred from matter at a higher temperature to matter at a lower temperature. • Heat is thermal energy moving from a warmer object to a cooler object. • Ice cubes melt in your hand because thermal energy is transferred from your hand to the ice cube. • Work and heat are both energy transfers.
Energy Temperature, Thermal Energy and Heat Specific Heat • A material with a high specific heat can absorb a great deal of thermal energy without a great change in temperature.
Energy Specific Heat • Specific Heat – the amount of energy required to raise the temperature of 1 kilogram of material by 1 Kelvin. • When an object is heated, its temperature rises. The temperature does not rise at the same rate for all objects. • The unit of measure for specific heat is joules per kilogram-kelvin or J/(kg • K)

Energy Specific Heat • Water requires more heat to raise its temperature than sand does. • At the beach, the sand may feel very hot, but the water may feel very cold.

Energy Specific Heat • A material with a high specific heat can absorb a great deal of thermal energy without a great change in temperature. • The energy gained or lost by a material is related to its mass, change in temperature, and specific heat. • You can calculate thermal energy changes with the following formula:
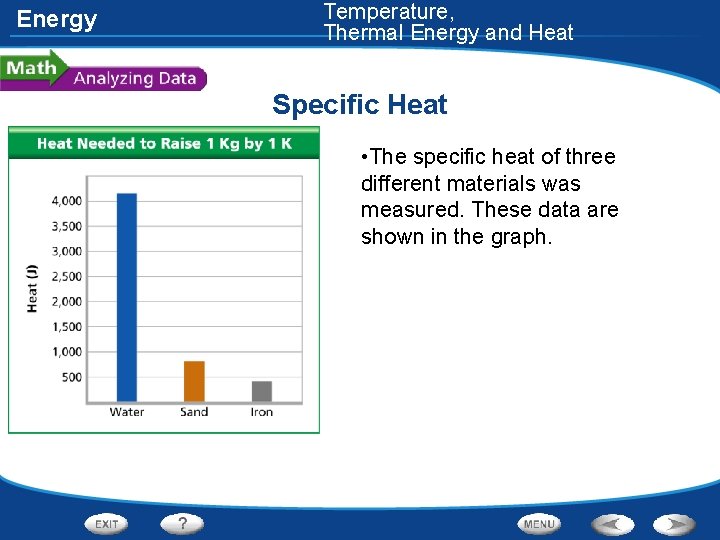
Energy Temperature, Thermal Energy and Heat Specific Heat • The specific heat of three different materials was measured. These data are shown in the graph.
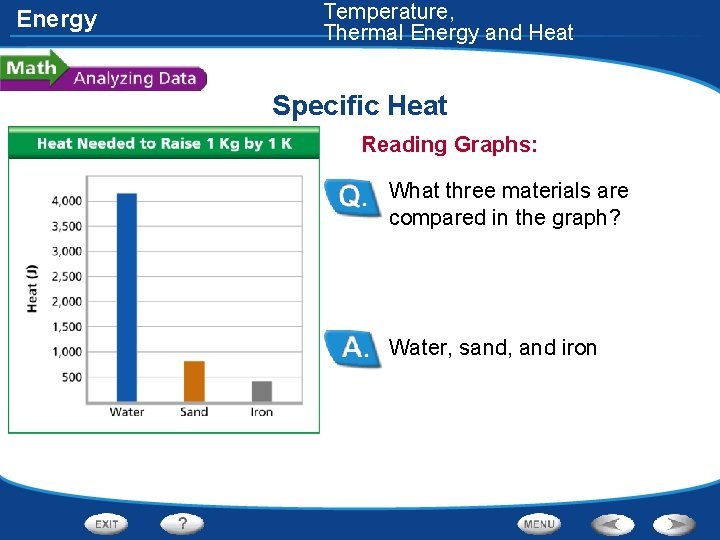
Energy Temperature, Thermal Energy and Heat Specific Heat Reading Graphs: What three materials are compared in the graph? Water, sand, and iron
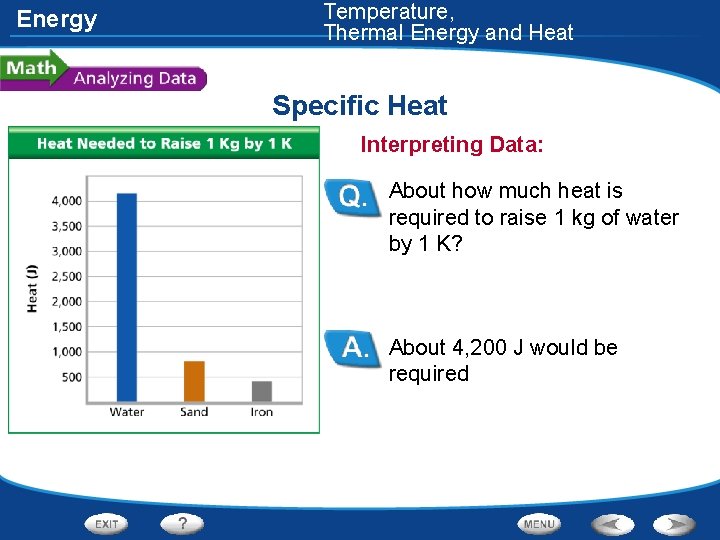
Energy Temperature, Thermal Energy and Heat Specific Heat Interpreting Data: About how much heat is required to raise 1 kg of water by 1 K? About 4, 200 J would be required
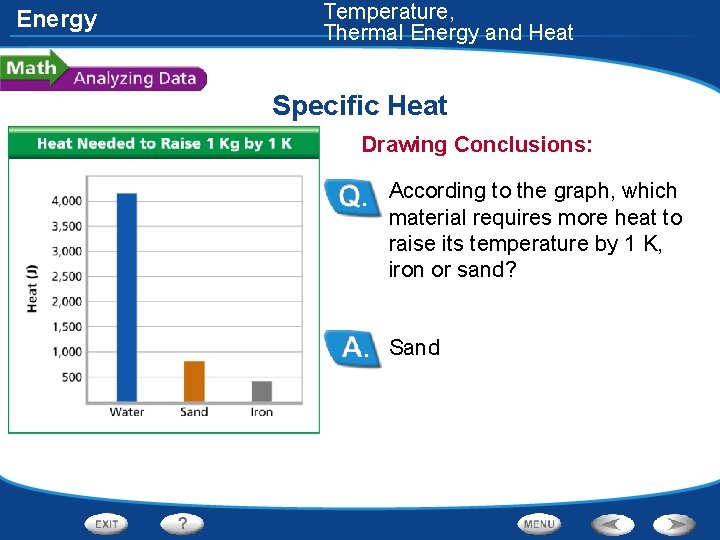
Energy Temperature, Thermal Energy and Heat Specific Heat Drawing Conclusions: According to the graph, which material requires more heat to raise its temperature by 1 K, iron or sand? Sand
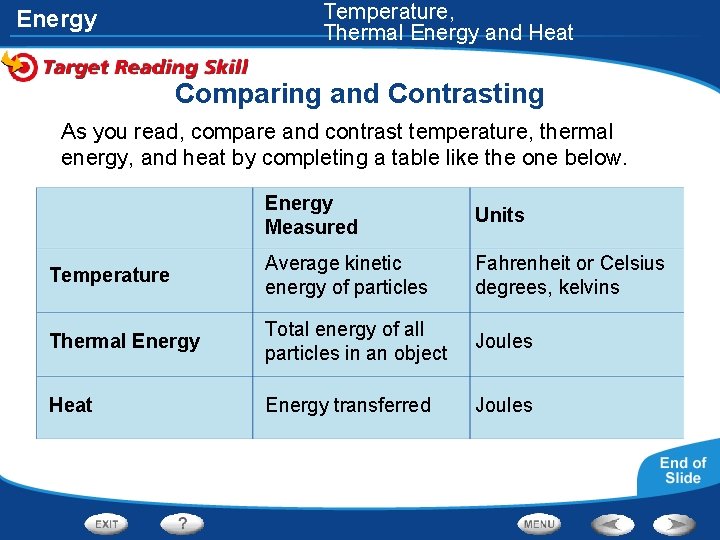
Temperature, Thermal Energy and Heat Energy Comparing and Contrasting As you read, compare and contrast temperature, thermal energy, and heat by completing a table like the one below. Energy Measured Units Temperature Average kinetic energy of particles Fahrenheit or Celsius degrees, kelvins Thermal Energy Total energy of all particles in an object Joules Heat Energy transferred Joules

Energy Temperature, Thermal Energy and Heat Temperature Click the Video button to watch a movie about temperature.
Energy Temperature, Thermal Energy and Heat Links on Temperature and Heat Click the Sci. Links button for links on temperature and heat.
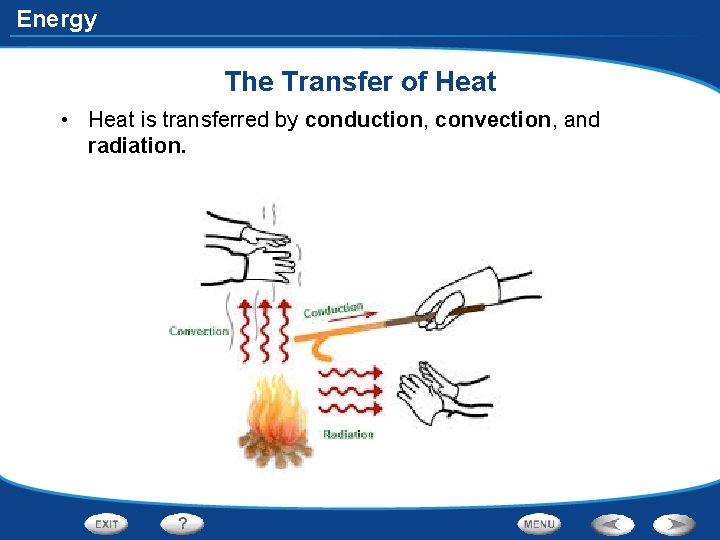
Energy The Transfer of Heat • Heat is transferred by conduction, convection, and radiation.
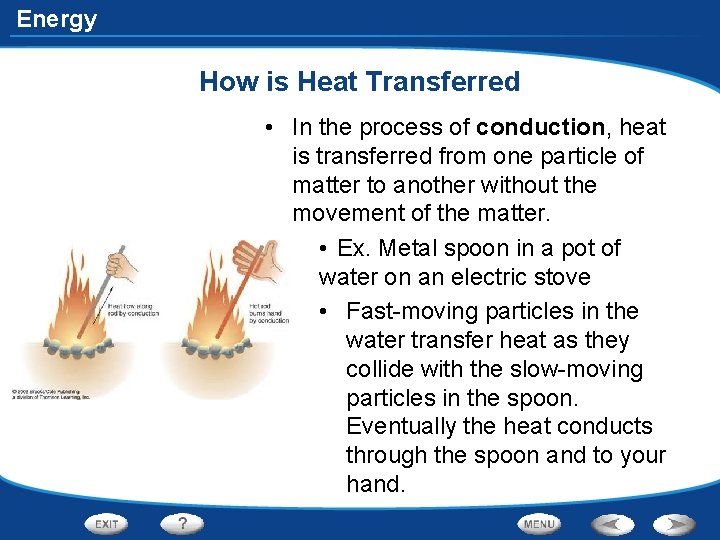
Energy How is Heat Transferred • In the process of conduction, heat is transferred from one particle of matter to another without the movement of the matter. • Ex. Metal spoon in a pot of water on an electric stove • Fast-moving particles in the water transfer heat as they collide with the slow-moving particles in the spoon. Eventually the heat conducts through the spoon and to your hand.
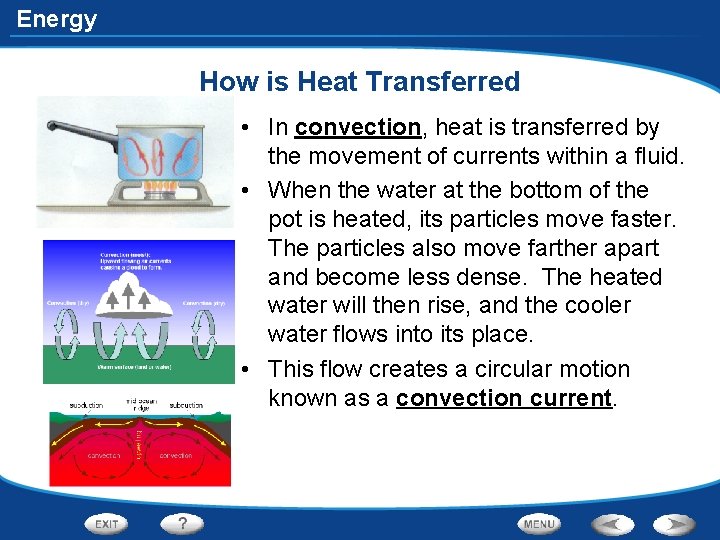
Energy How is Heat Transferred • In convection, heat is transferred by the movement of currents within a fluid. • When the water at the bottom of the pot is heated, its particles move faster. The particles also move farther apart and become less dense. The heated water will then rise, and the cooler water flows into its place. • This flow creates a circular motion known as a convection current.
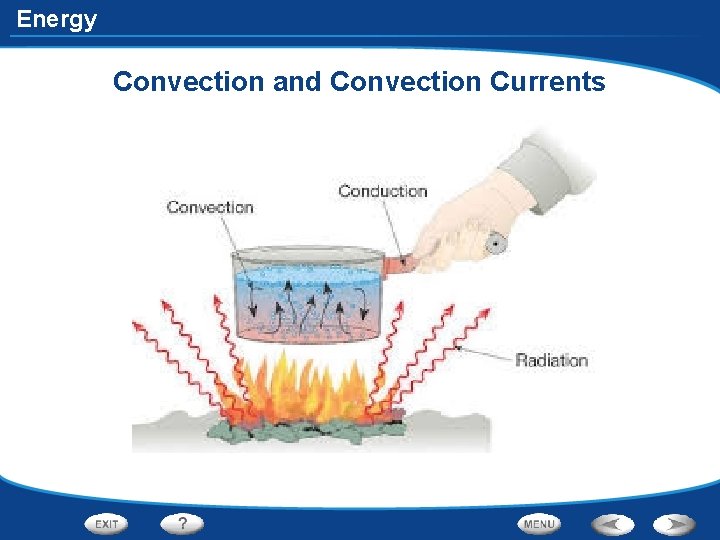
Energy Convection and Convection Currents
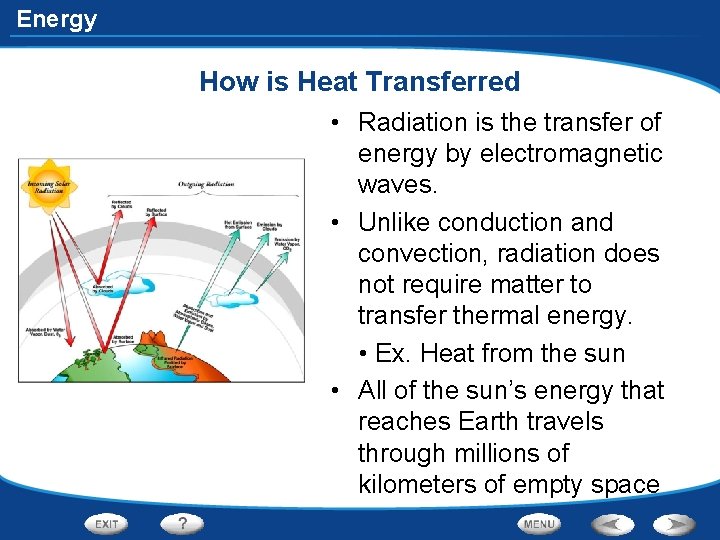
Energy How is Heat Transferred • Radiation is the transfer of energy by electromagnetic waves. • Unlike conduction and convection, radiation does not require matter to transfer thermal energy. • Ex. Heat from the sun • All of the sun’s energy that reaches Earth travels through millions of kilometers of empty space
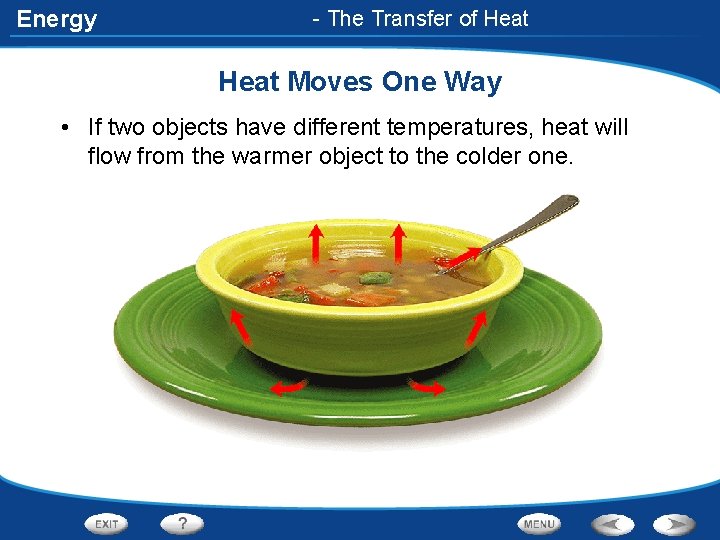
Energy - The Transfer of Heat Moves One Way • If two objects have different temperatures, heat will flow from the warmer object to the colder one.
Energy Heat Moves One Way • When heat flows into matter, thermal energy of the matter increases • As thermal energy increases, the temperature increases • Heat will flow from one object to the other until the two objects have the same temperature • Ex. Hot soup cooling to room temperature
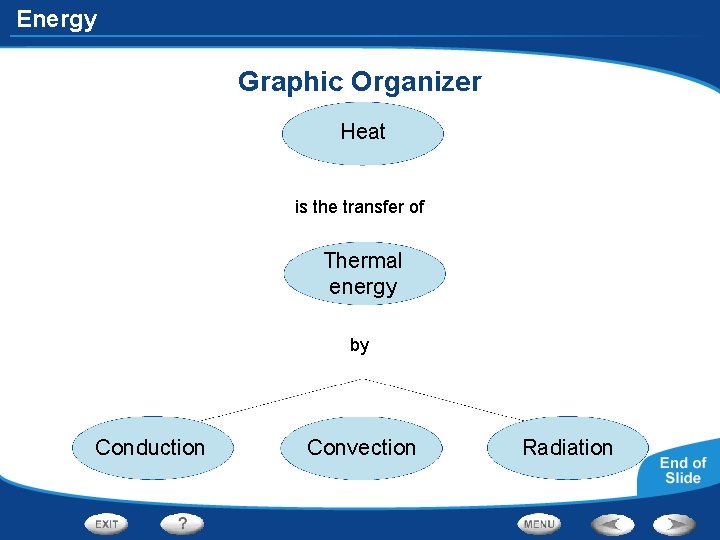
Energy Graphic Organizer Heat is the transfer of Thermal energy by Conduction Convection Radiation

Energy Heat Moves One Way • Ice does not transfer cold to other materials • There is no such thing as “coldness” • Heat transfer occurs in only one direction • Heat transferring to ice will melt the ice

Energy Conductors and Insulators • A material can be either a conductor or an insulator • A conductor transfers thermal energy well. An insulator does not transfer thermal energy well. • What materials do you think would be considered a conductor? • What materials do you think would be considered an insulator?

Energy Conductors and Insulators • A metal spatula would transfer heat better than a plastic spatula • A material that conducts heat well is called a conductor • Ex. Metals such as silver and stainless steel • Good conductors, such as a tile floor, feel cool to the touch because it easily transfers heat away from your skin

Energy Conductors and Insulators • A material that does not conduct heat well is called an insulator • Ex. Wood, wool, paper, and gases in the air • Clothes and blankets are insulators that slow the transfer of heat out of your body • Insulation in a building prevents heat from entering the building in hot weather and from escaping in cold weather.
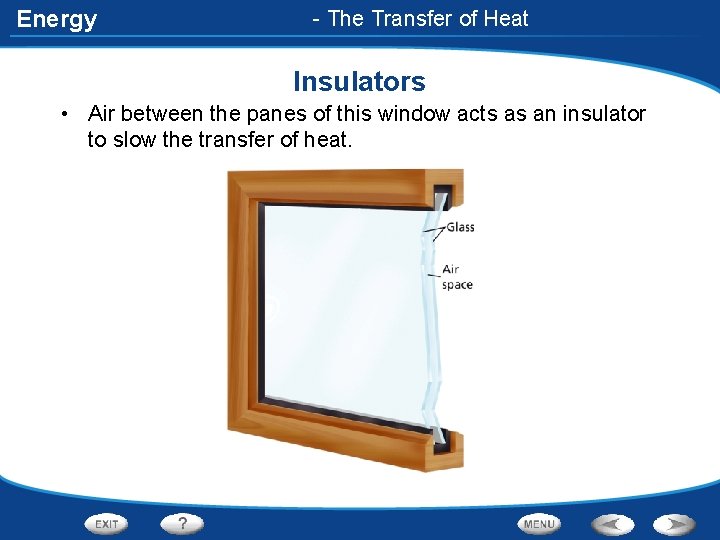
Energy - The Transfer of Heat Insulators • Air between the panes of this window acts as an insulator to slow the transfer of heat.

Energy - The Transfer of Heat Links on Heat Transfer Click the Sci. Links button for links on forms of heat transfer.
Energy - The Transfer of Heat Insulators Click the Video button to watch a movie about insulators.

Energy Thermal Energy and States of Matter • Most matter on Earth can exist in three states–solid, liquid, and gas.
Energy Solids • Particles that make up a solid are packed together in relatively fixed positions • These particles cannot move out of their positions but can only vibrate back and forth • This is why solids retain a fixed shape and volume

Energy Liquids • Particles that make up a liquid are close together, but they are not held together as tightly as those of a solid • Because these particles can move around, liquids don’t have a definite shape • Liquids do have a definite volume

Energy Gases • In gases, particles are moving so fast that they don’t even stay close together • Gases expand to fill all the space available • Gases do not have a fixed shape or volume
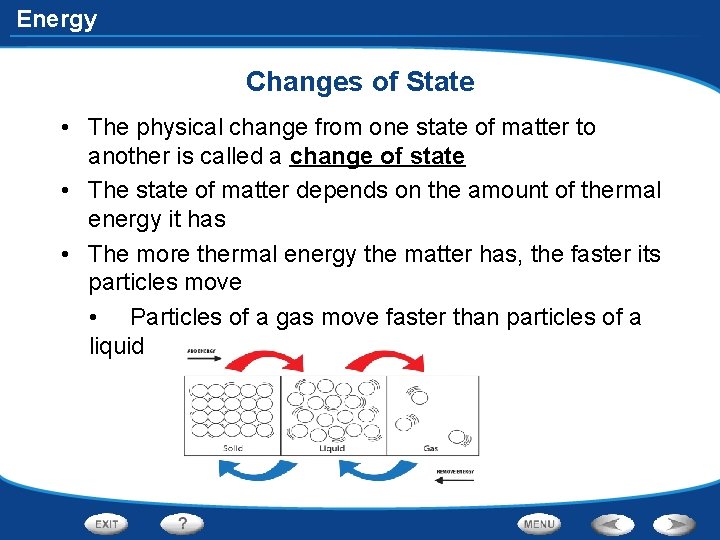
Energy Changes of State • The physical change from one state of matter to another is called a change of state • The state of matter depends on the amount of thermal energy it has • The more thermal energy the matter has, the faster its particles move • Particles of a gas move faster than particles of a liquid
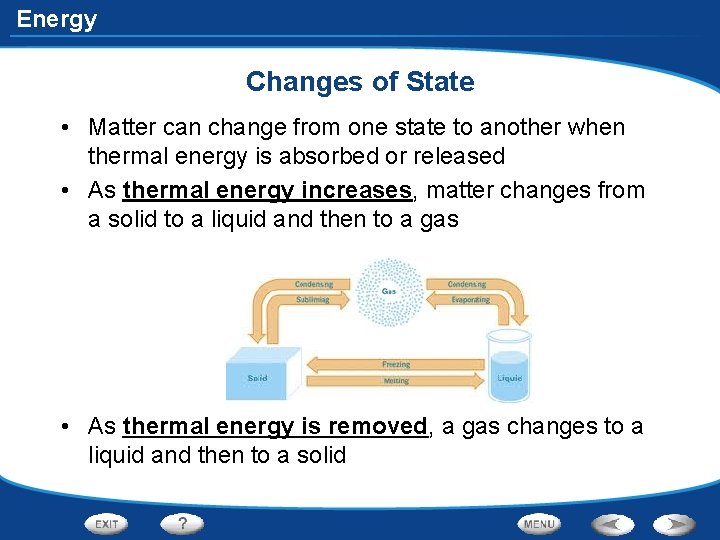
Energy Changes of State • Matter can change from one state to another when thermal energy is absorbed or released • As thermal energy increases, matter changes from a solid to a liquid and then to a gas • As thermal energy is removed, a gas changes to a liquid and then to a solid
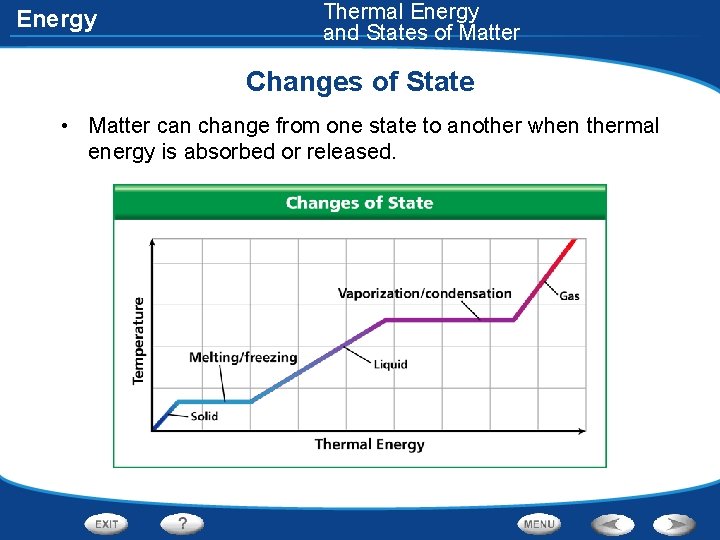
Energy Thermal Energy and States of Matter Changes of State • Matter can change from one state to another when thermal energy is absorbed or released.
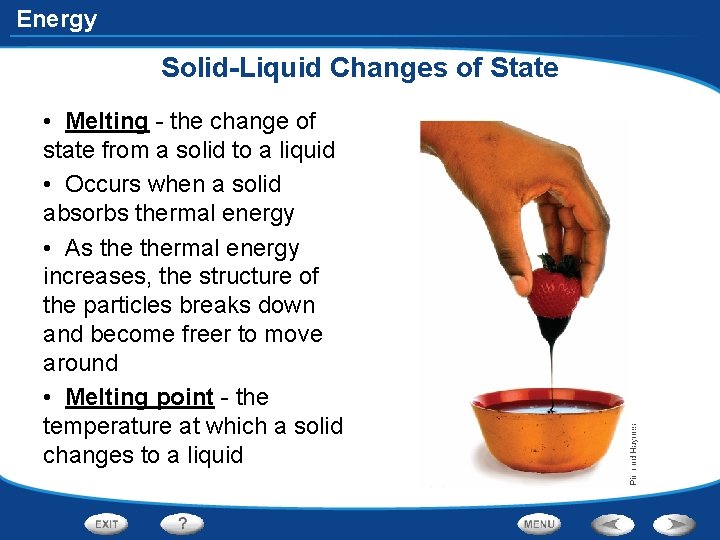
Energy Solid-Liquid Changes of State • Melting - the change of state from a solid to a liquid • Occurs when a solid absorbs thermal energy • As thermal energy increases, the structure of the particles breaks down and become freer to move around • Melting point - the temperature at which a solid changes to a liquid
Energy Solid-Liquid Changes of State • Freezing- the change of state from a liquid to a solid • Occurs when matter releases thermal energy • Freezing point- the temperature at which matter changes from a liquid to a solid • For a given type of matter, the freezing point and melting point are the same • The difference between the two is whether the matter is gaining or releasing thermal energy • Ex. Water freezing/melting at 32°Fahrenheit
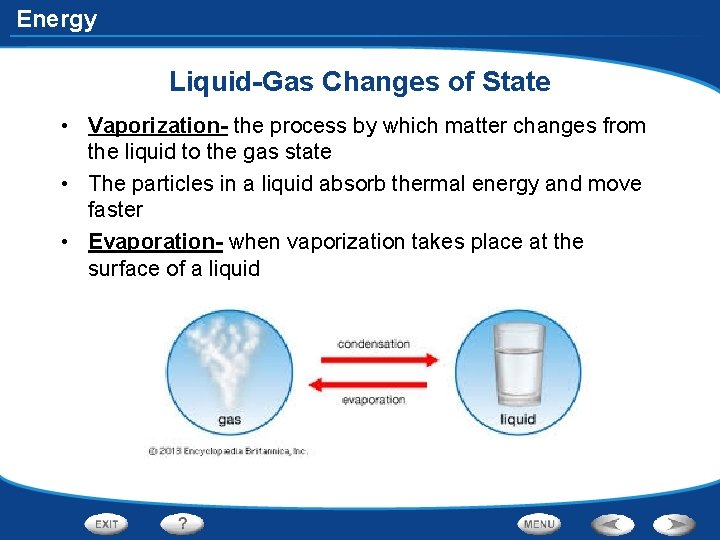
Energy Liquid-Gas Changes of State • Vaporization- the process by which matter changes from the liquid to the gas state • The particles in a liquid absorb thermal energy and move faster • Evaporation- when vaporization takes place at the surface of a liquid

Energy Liquid-Gas Changes of State • Boiling- when vaporization occurs below the surface of a liquid due to higher temperatures • Boiling point- temperature at which a liquid boils • Condensation- a change from the gas state to the liquid state • Occurs when a gas loses a certain amount of thermal energy
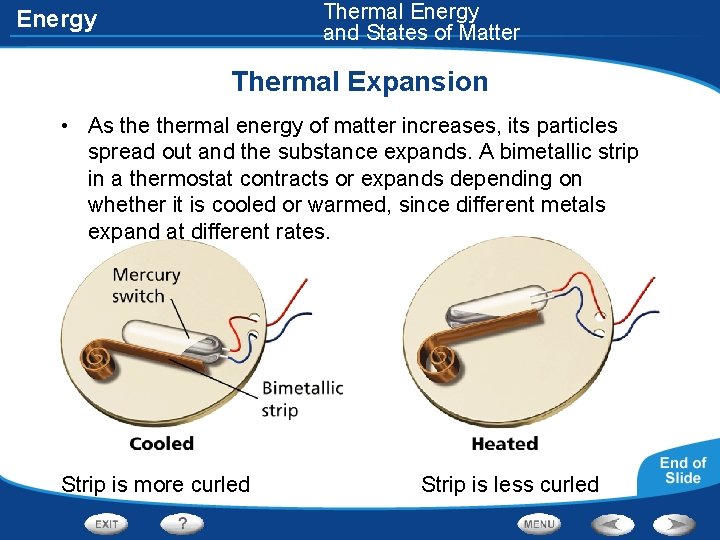
Thermal Energy and States of Matter Energy Thermal Expansion • As thermal energy of matter increases, its particles spread out and the substance expands. A bimetallic strip in a thermostat contracts or expands depending on whether it is cooled or warmed, since different metals expand at different rates. Strip is more curled Strip is less curled

Energy Thermal Expansion • Thermal expansion- the expanding of matter when it is heated • Ex. The lid of a tight jar loosening when run under hot water • When matter is cooled, thermal energy is released and the particles slow down and move closer together. • As matter is cooled, it contracts, or decreases in volume • In bimetallic strips in thermostats, as one side expands more than the other, the movement of the strip operates a switch that can turn a heating system on or off.

Energy Thermal Energy and States of Matter Links on Changes of State Click the Sci. Links button for links on changes of state.
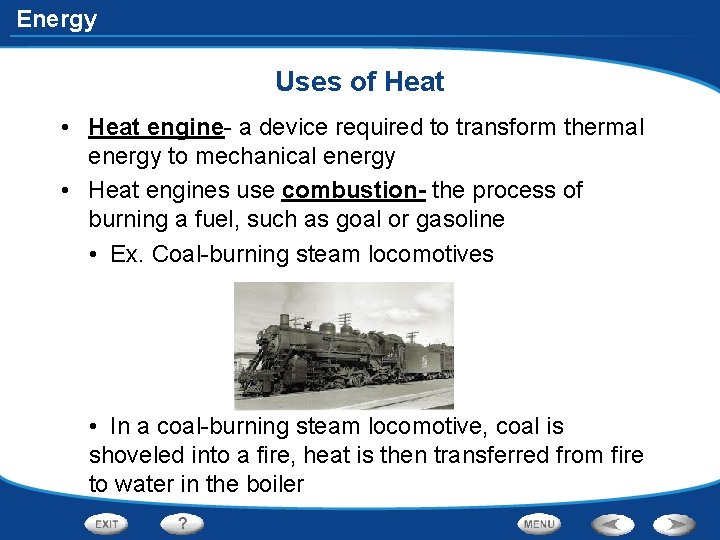
Energy Uses of Heat • Heat engine- a device required to transform thermal energy to mechanical energy • Heat engines use combustion- the process of burning a fuel, such as goal or gasoline • Ex. Coal-burning steam locomotives • In a coal-burning steam locomotive, coal is shoveled into a fire, heat is then transferred from fire to water in the boiler
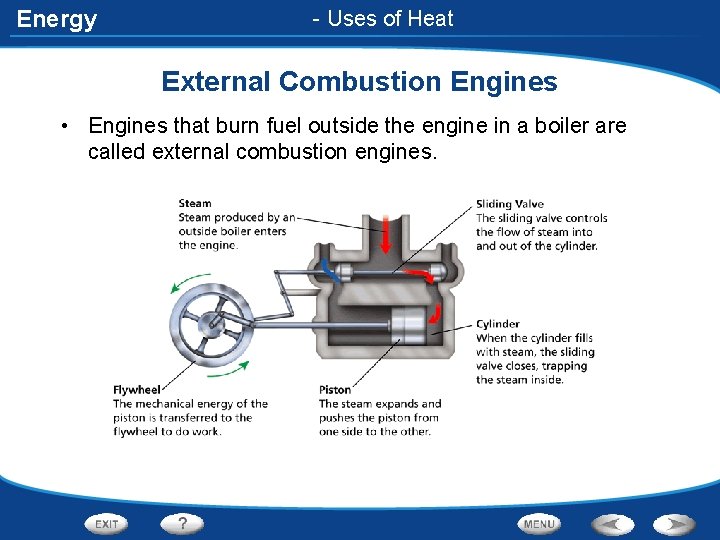
Energy - Uses of Heat External Combustion Engines • Engines that burn fuel outside the engine in a boiler are called external combustion engines.
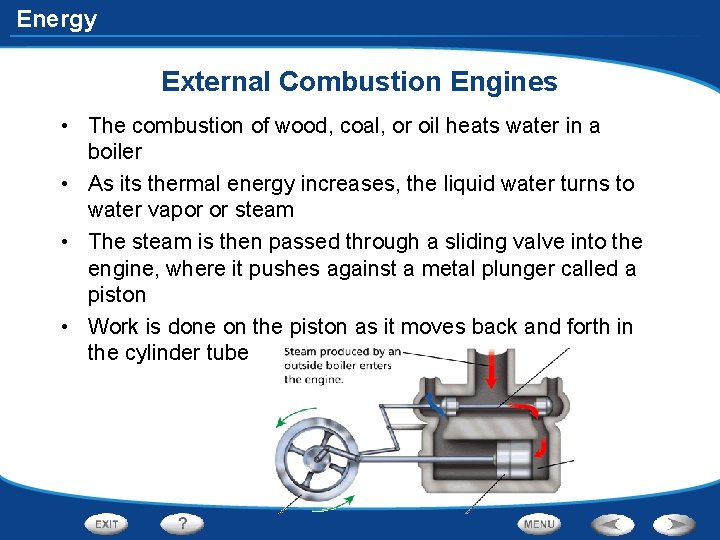
Energy External Combustion Engines • The combustion of wood, coal, or oil heats water in a boiler • As its thermal energy increases, the liquid water turns to water vapor or steam • The steam is then passed through a sliding valve into the engine, where it pushes against a metal plunger called a piston • Work is done on the piston as it moves back and forth in the cylinder tube
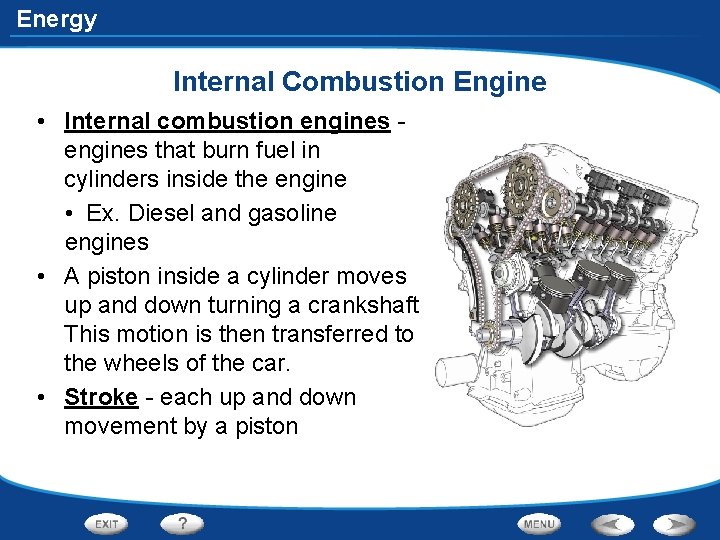
Energy Internal Combustion Engine • Internal combustion engines that burn fuel in cylinders inside the engine • Ex. Diesel and gasoline engines • A piston inside a cylinder moves up and down turning a crankshaft. This motion is then transferred to the wheels of the car. • Stroke - each up and down movement by a piston
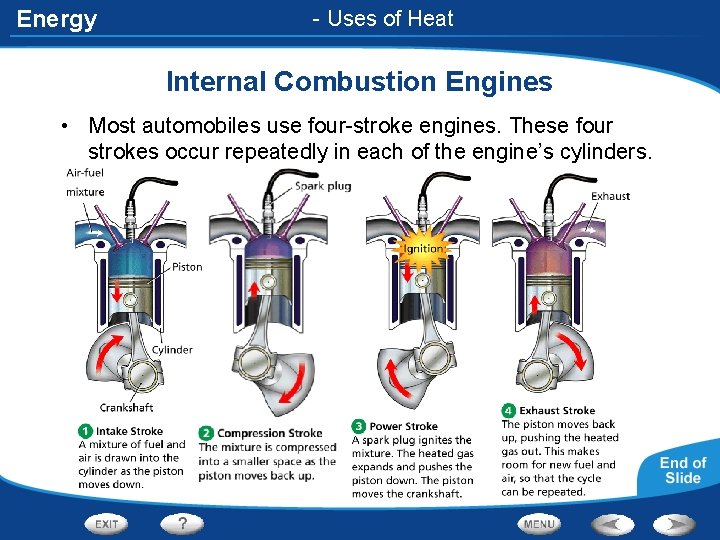
Energy - Uses of Heat Internal Combustion Engines • Most automobiles use four-stroke engines. These four strokes occur repeatedly in each of the engine’s cylinders.

Energy - Uses of Heat Four-Stroke Engine Activity Click the Active Art button to open a browser window and access Active Art about four-stroke engines.
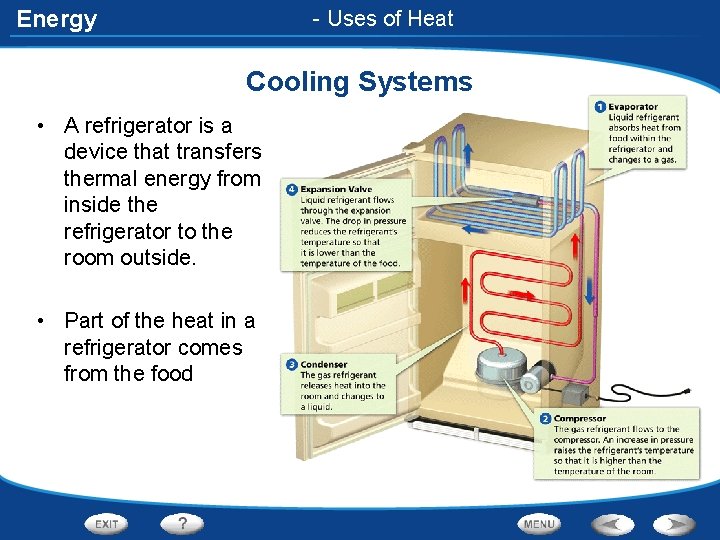
Energy - Uses of Heat Cooling Systems • A refrigerator is a device that transfers thermal energy from inside the refrigerator to the room outside. • Part of the heat in a refrigerator comes from the food
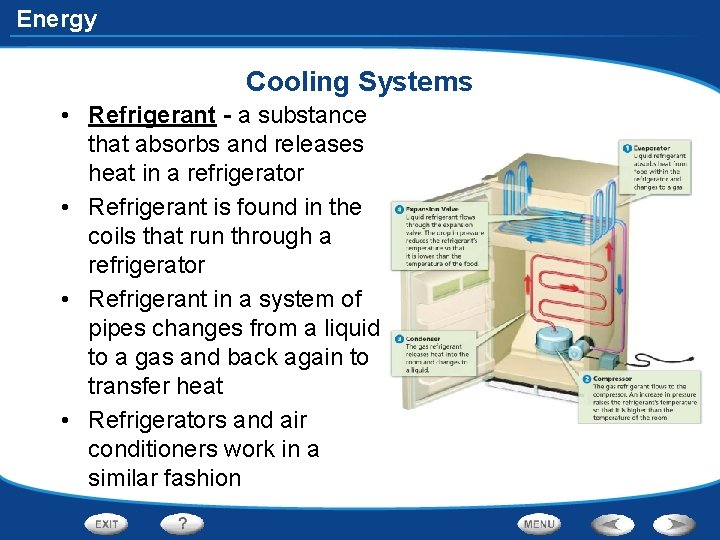
Energy Cooling Systems • Refrigerant - a substance that absorbs and releases heat in a refrigerator • Refrigerant is found in the coils that run through a refrigerator • Refrigerant in a system of pipes changes from a liquid to a gas and back again to transfer heat • Refrigerators and air conditioners work in a similar fashion
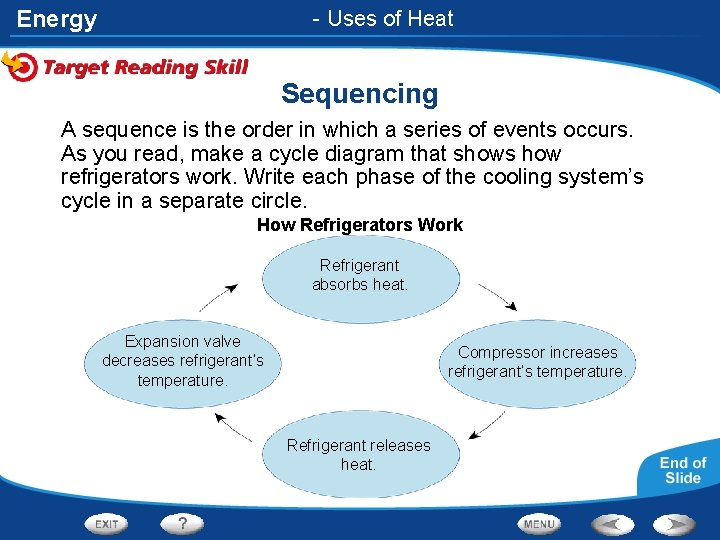
Energy - Uses of Heat Sequencing A sequence is the order in which a series of events occurs. As you read, make a cycle diagram that shows how refrigerators work. Write each phase of the cooling system’s cycle in a separate circle. How Refrigerators Work Refrigerant absorbs heat. Expansion valve decreases refrigerant’s temperature. Compressor increases refrigerant’s temperature. Refrigerant releases heat.
- Slides: 59