Temperature Heat Transfer Thermal Energy What is heat






- Slides: 14

Temperature, Heat Transfer & Thermal Energy

What is heat? What is temperature?

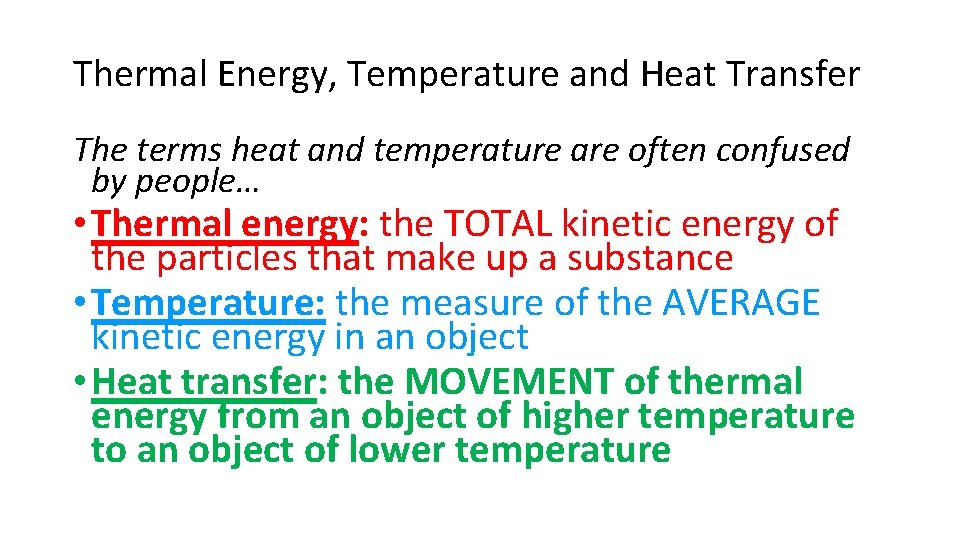
Thermal Energy, Temperature and Heat Transfer The terms heat and temperature are often confused by people… • Thermal energy: the TOTAL kinetic energy of the particles that make up a substance • Temperature: the measure of the AVERAGE kinetic energy in an object • Heat transfer: the MOVEMENT of thermal energy from an object of higher temperature to an object of lower temperature

Remember… • There is no such thing as “cold” just more heat and less heat • Heat likes to go from high to low until it has nowhere else to go! • We call that EQUILIBRIUM

Coffee Pot or the North Sea? Which is hotter? (temperature) Which contains more energy? (heat)

Iceberg or a glass of water? Which is hotter? (temperature) Which contains more energy? (heat)

…something which has a higher temperature than another does not necessarily contain more heat energy… This Photo by Unknown Author is licensed under CC BY-SA-NC

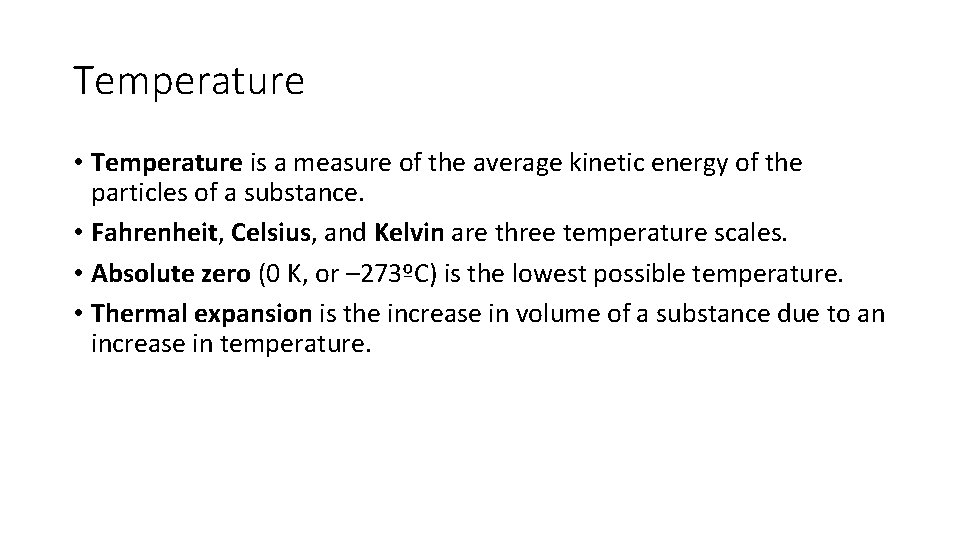
Temperature • Temperature is a measure of the average kinetic energy of the particles of a substance. • Fahrenheit, Celsius, and Kelvin are three temperature scales. • Absolute zero (0 K, or – 273ºC) is the lowest possible temperature. • Thermal expansion is the increase in volume of a substance due to an increase in temperature.

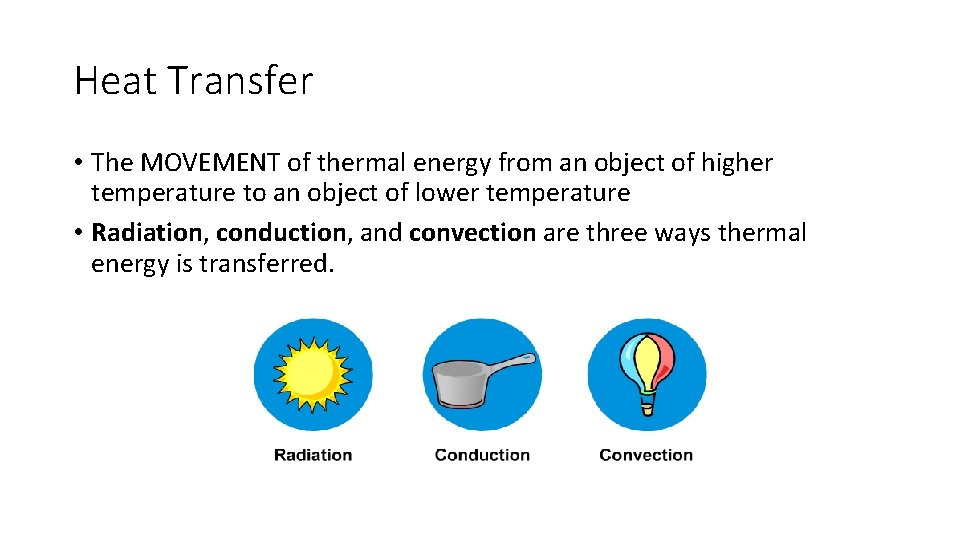
Heat Transfer • The MOVEMENT of thermal energy from an object of higher temperature to an object of lower temperature • Radiation, conduction, and convection are three ways thermal energy is transferred.

Radiation • The transfer of energy as electromagnetic waves • Does NOT require a medium and CAN occur in a vacuum • Energy moves between molecules • Example: heat radiating from the sun; heat radiating from a lamp or a fire

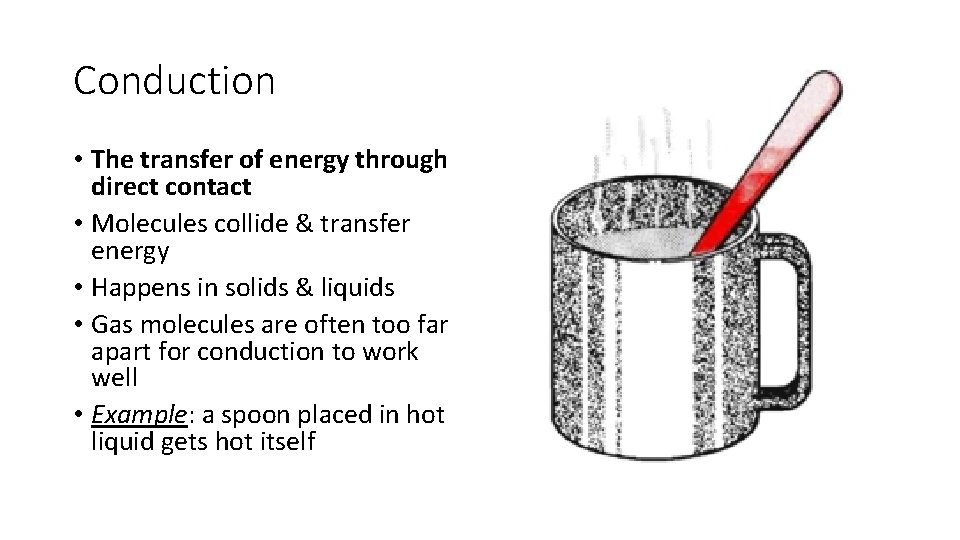
Conduction • The transfer of energy through direct contact • Molecules collide & transfer energy • Happens in solids & liquids • Gas molecules are often too far apart for conduction to work well • Example: a spoon placed in hot liquid gets hot itself

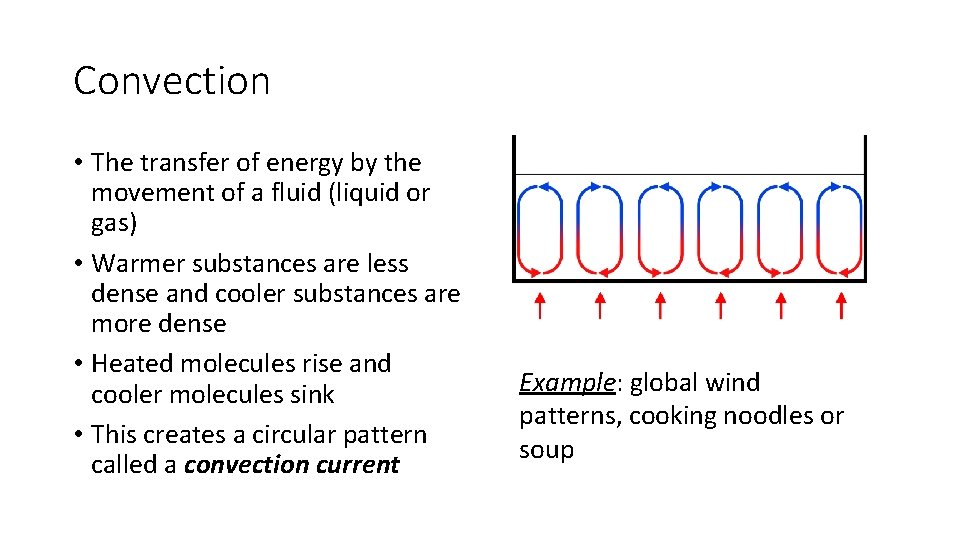
Convection • The transfer of energy by the movement of a fluid (liquid or gas) • Warmer substances are less dense and cooler substances are more dense • Heated molecules rise and cooler molecules sink • This creates a circular pattern called a convection current Example: global wind patterns, cooking noodles or soup

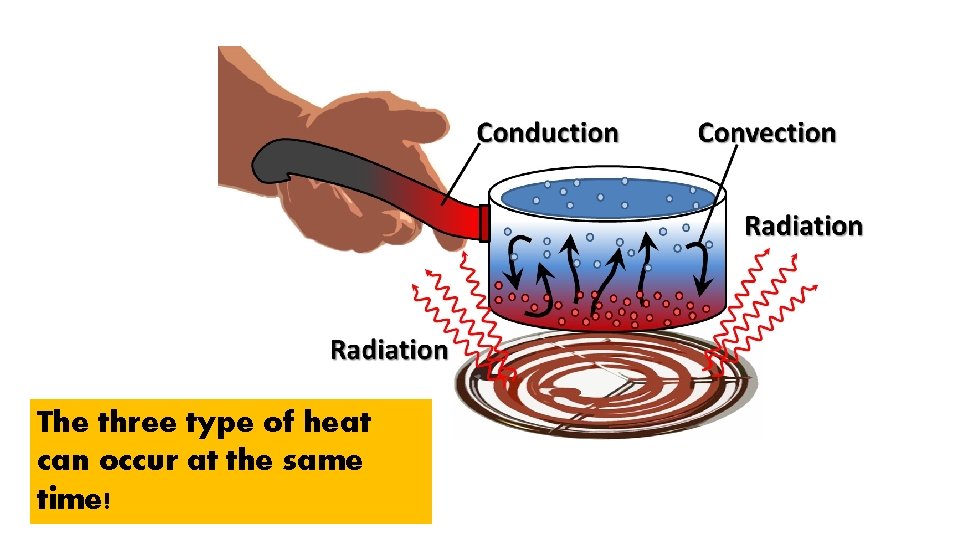
The three type of heat can occur at the same time!

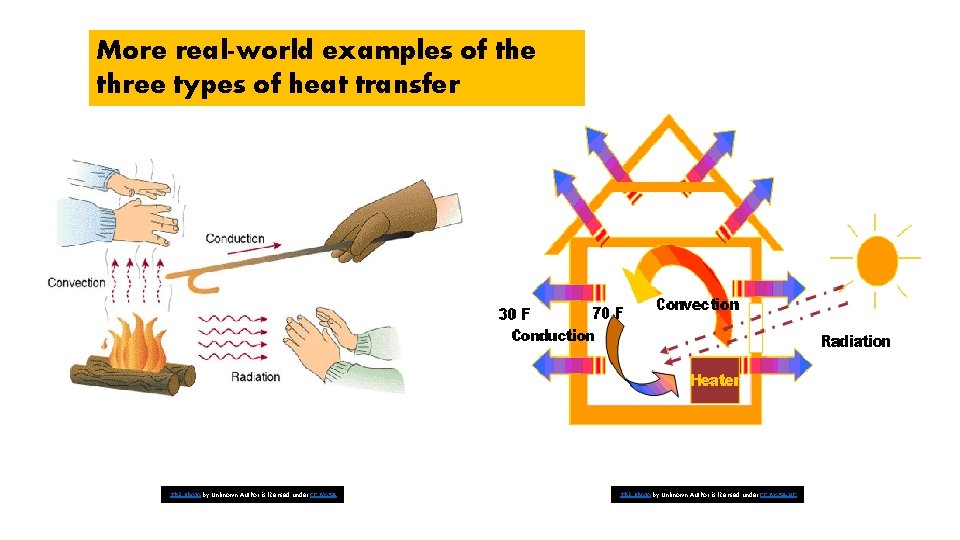
More real-world examples of the three types of heat transfer This Photo by Unknown Author is licensed under CC BY-SA-NC