Heat Temperature Heat Temperature Heat is a form







- Slides: 28

Heat & Temperature

Heat & Temperature �Heat is a form of Energy. �It is measured in joules (J) �Temperature is a measure of how hot or cold something is. �It is measured in degree Celcius (°C)

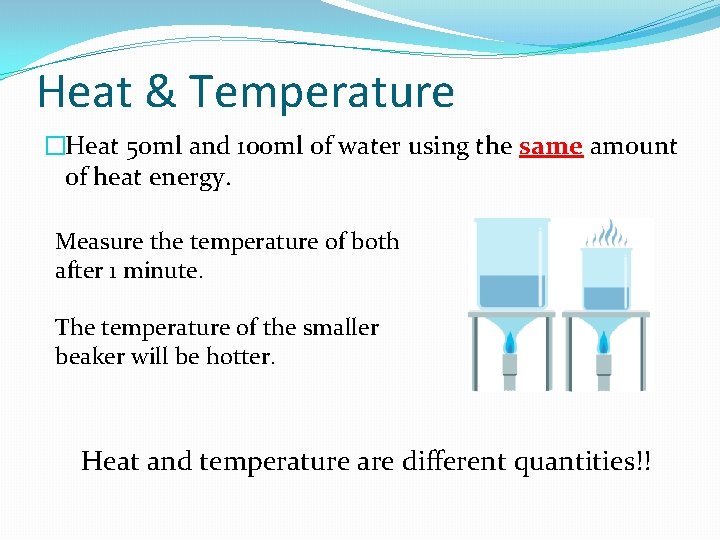
Heat & Temperature �Heat 50 ml and 100 ml of water using the same amount of heat energy. Measure the temperature of both after 1 minute. The temperature of the smaller beaker will be hotter. Heat and temperature are different quantities!!

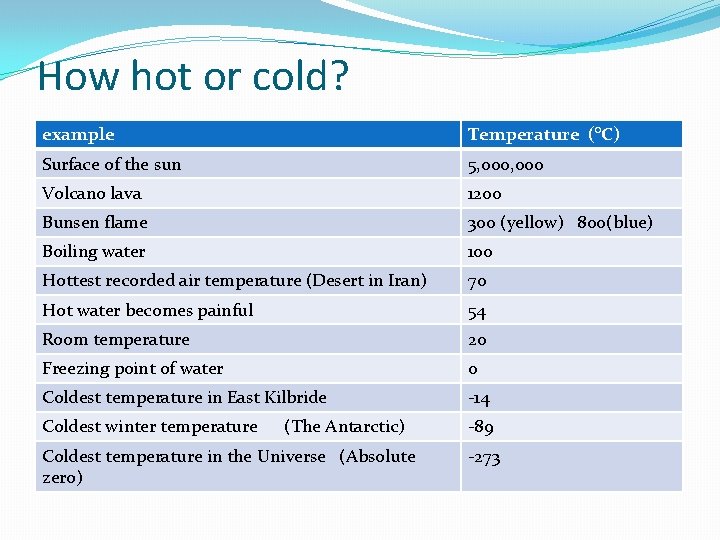
How hot or cold? example Temperature (°C) Surface of the sun 5, 000 Volcano lava 1200 Bunsen flame 300 (yellow) 800(blue) Boiling water 100 Hottest recorded air temperature (Desert in Iran) 70 Hot water becomes painful 54 Room temperature 20 Freezing point of water 0 Coldest temperature in East Kilbride -14 Coldest winter temperature -89 (The Antarctic) Coldest temperature in the Universe (Absolute zero) -273

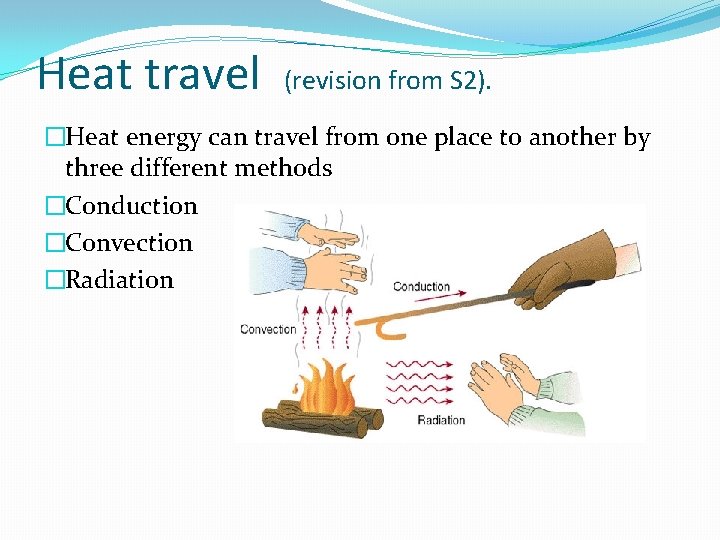
Heat travel (revision from S 2). �Heat energy can travel from one place to another by three different methods �Conduction �Convection �Radiation

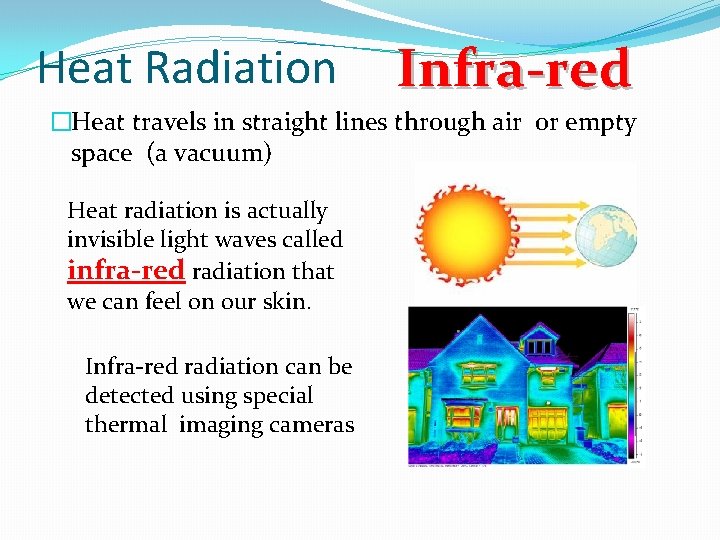
Heat Radiation Infra-red �Heat travels in straight lines through air or empty space (a vacuum) Heat radiation is actually invisible light waves called infra-red radiation that we can feel on our skin. Infra-red radiation can be detected using special thermal imaging cameras

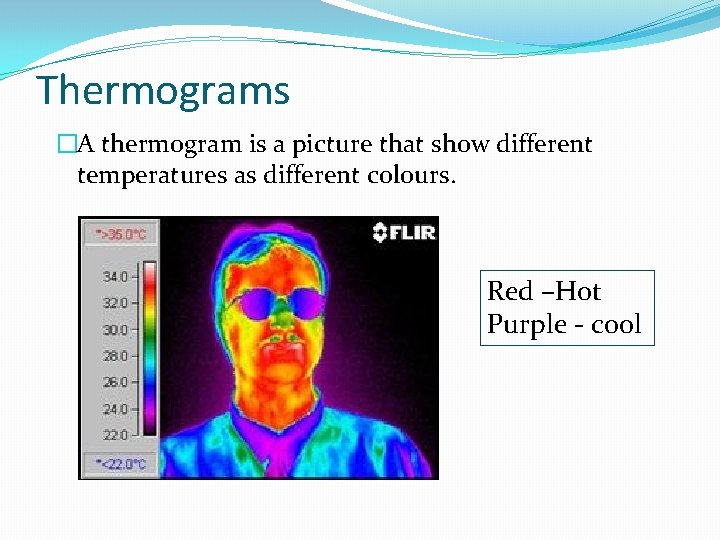
Thermograms �A thermogram is a picture that show different temperatures as different colours. Red –Hot Purple - cool

Specific Heat capacity �It takes a specific amount of heat energy to heat 1 kg of a substance by 1°C �This amount of heat energy is called the specific heat capacity of the substance. Symbol : c �Every substance has a different value for c. For example : it takes 4180 J of heat energy to heat 1 kg of water by 1°C specific heat capacity of water = 4180 J kg-1 °C-1

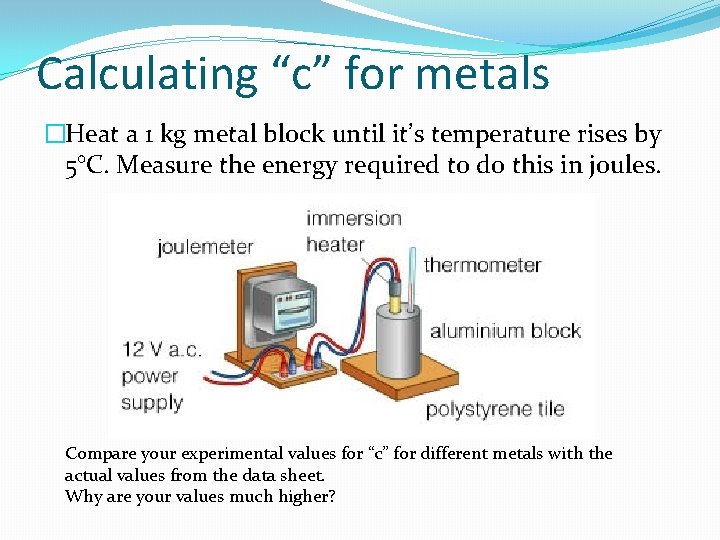
Calculating “c” for metals �Heat a 1 kg metal block until it’s temperature rises by 5°C. Measure the energy required to do this in joules. Compare your experimental values for “c” for different metals with the actual values from the data sheet. Why are your values much higher?

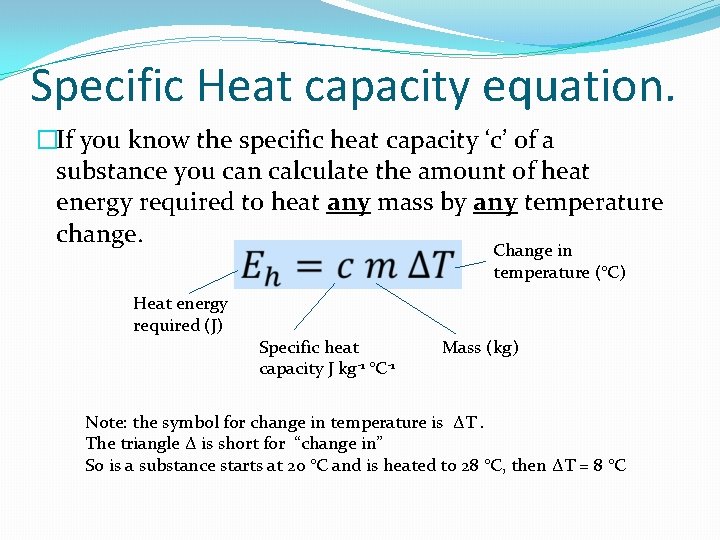
Specific Heat capacity equation. �If you know the specific heat capacity ‘c’ of a substance you can calculate the amount of heat energy required to heat any mass by any temperature change. Change in temperature (°C) Heat energy required (J) Specific heat capacity J kg-1 °C-1 Mass (kg) Note: the symbol for change in temperature is ΔT. The triangle Δ is short for “change in” So is a substance starts at 20 °C and is heated to 28 °C, then ΔT = 8 °C


examples � 1. Calculate the heat energy required to heat 20 kg of copper from 20 °C to 80 °C � 2. A 8 kg block of aluminium sitting outside in the sun absorbs 86, 600 J of heat energy from the sun. Calculate the temperature rise of the block. 3. An iron horseshoe is heated in a furnace. Its temperature increases by 600 °C when it absorbs 144 000 J of heat energy. Calculate the mass of the horseshoe.

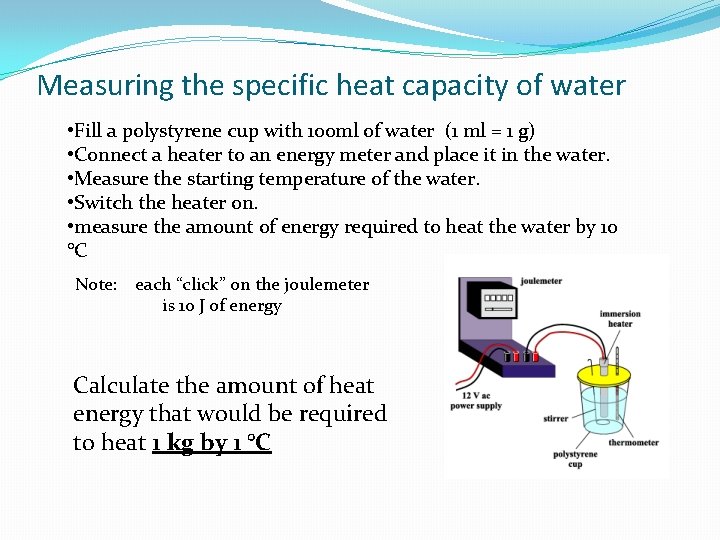
Measuring the specific heat capacity of water • Fill a polystyrene cup with 100 ml of water (1 ml = 1 g) • Connect a heater to an energy meter and place it in the water. • Measure the starting temperature of the water. • Switch the heater on. • measure the amount of energy required to heat the water by 10 °C Note: each “click” on the joulemeter is 10 J of energy Calculate the amount of heat energy that would be required to heat 1 kg by 1 °C

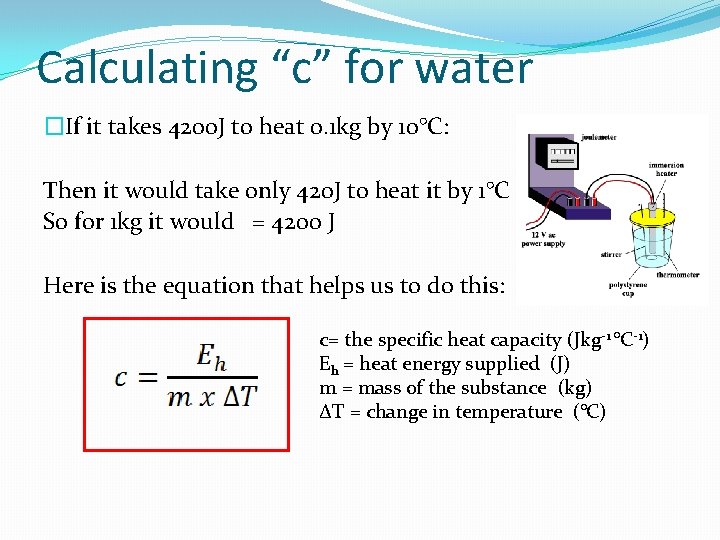
Calculating “c” for water �If it takes 4200 J to heat 0. 1 kg by 10°C: Then it would take only 420 J to heat it by 1°C So for 1 kg it would = 4200 J Here is the equation that helps us to do this: c= the specific heat capacity (Jkg-1 °C-1) Eh = heat energy supplied (J) m = mass of the substance (kg) ΔT = change in temperature (°C)

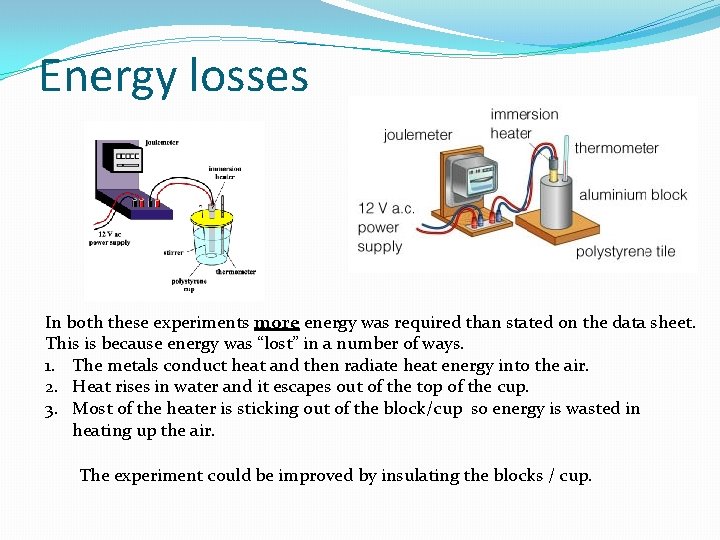
Energy losses In both these experiments more energy was required than stated on the data sheet. This is because energy was “lost” in a number of ways. 1. The metals conduct heat and then radiate heat energy into the air. 2. Heat rises in water and it escapes out of the top of the cup. 3. Most of the heater is sticking out of the block/cup so energy is wasted in heating up the air. The experiment could be improved by insulating the blocks / cup.

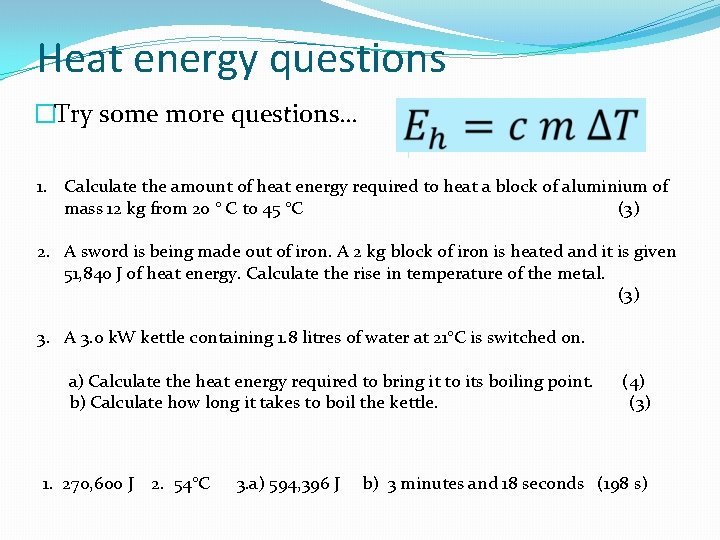
Heat energy questions �Try some more questions… 1. Calculate the amount of heat energy required to heat a block of aluminium of mass 12 kg from 20 ° C to 45 °C (3) 2. A sword is being made out of iron. A 2 kg block of iron is heated and it is given 51, 840 J of heat energy. Calculate the rise in temperature of the metal. (3) 3. A 3. 0 k. W kettle containing 1. 8 litres of water at 21°C is switched on. a) Calculate the heat energy required to bring it to its boiling point. b) Calculate how long it takes to boil the kettle. 1. 270, 600 J 2. 54°C 3. a) 594, 396 J (4) (3) b) 3 minutes and 18 seconds (198 s)

There’s a problem……… �If you keep heating a substance eventually you get to a point where it doesn’t get any hotter. It changes state!! (melts or evaporates!) So the equation Eh = cmΔT doesn’t work anymore! We need a new equation for when the state is changing!

State change �If you continue heating a substance it gets to a point where it doesn’t get any hotter but it changes state!

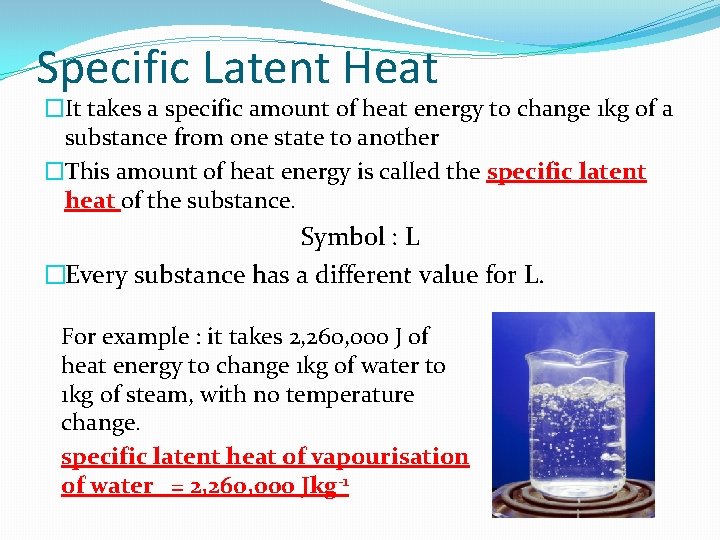
Specific Latent Heat �It takes a specific amount of heat energy to change 1 kg of a substance from one state to another �This amount of heat energy is called the specific latent heat of the substance. Symbol : L �Every substance has a different value for L. For example : it takes 2, 260, 000 J of heat energy to change 1 kg of water to 1 kg of steam, with no temperature change. specific latent heat of vapourisation of water = 2, 260, 000 Jkg-1

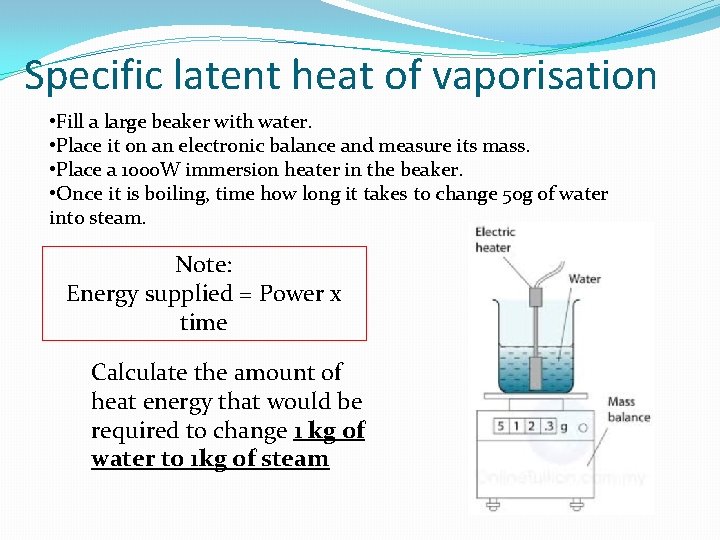
Specific latent heat of vaporisation • Fill a large beaker with water. • Place it on an electronic balance and measure its mass. • Place a 1000 W immersion heater in the beaker. • Once it is boiling, time how long it takes to change 50 g of water into steam. Note: Energy supplied = Power x time Calculate the amount of heat energy that would be required to change 1 kg of water to 1 kg of steam

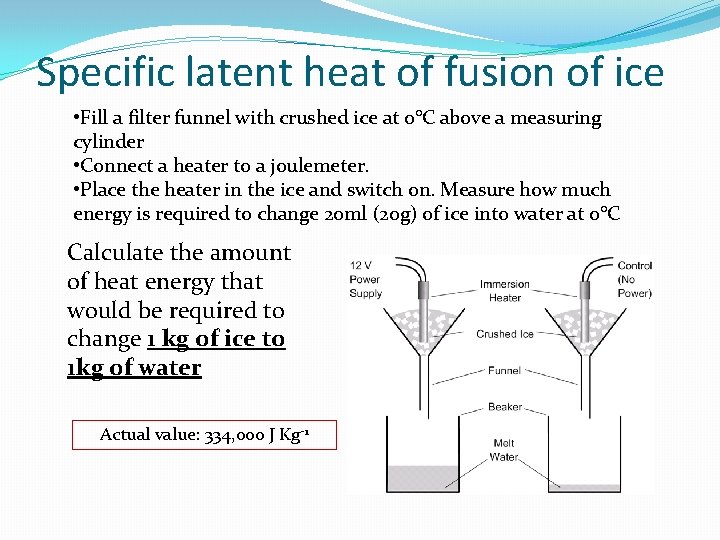
Specific latent heat of fusion of ice • Fill a filter funnel with crushed ice at 0°C above a measuring cylinder • Connect a heater to a joulemeter. • Place the heater in the ice and switch on. Measure how much energy is required to change 20 ml (20 g) of ice into water at 0°C Calculate the amount of heat energy that would be required to change 1 kg of ice to 1 kg of water Actual value: 334, 000 J Kg-1

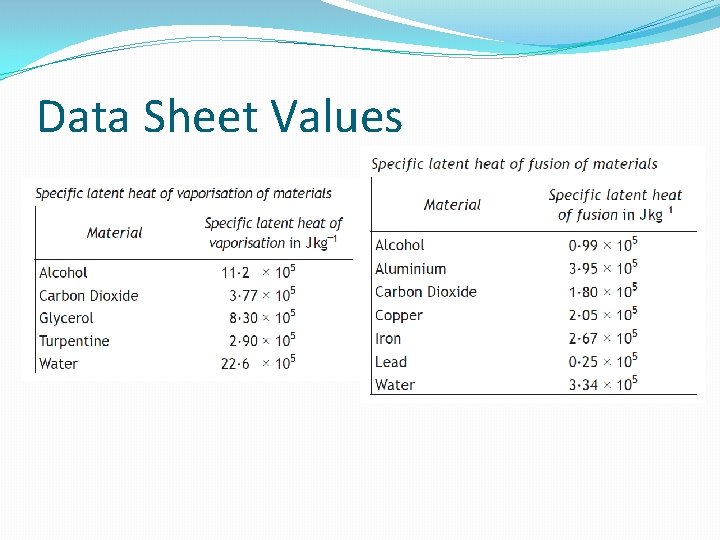
Data Sheet Values

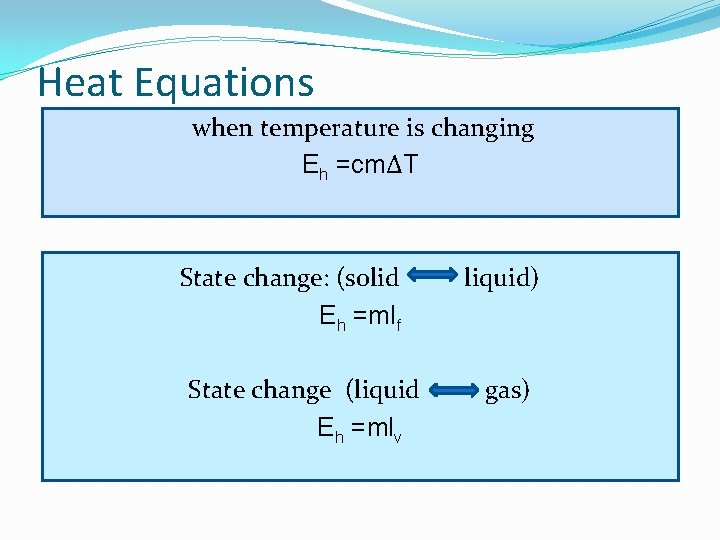
Heat Equations when temperature is changing Eh =cmΔT State change: (solid Eh =mlf State change (liquid Eh =mlv liquid) gas)

Heat calculation �A pot of boiling water is left on for 20 minutes and 1. 6 kg of water is changed into steam. �How much heat energy was used to do this? Answer: Eh = mlv = 1. 6 x 2, 260, 000 = 3, 616, 000 J = 3. 62 x 106 J

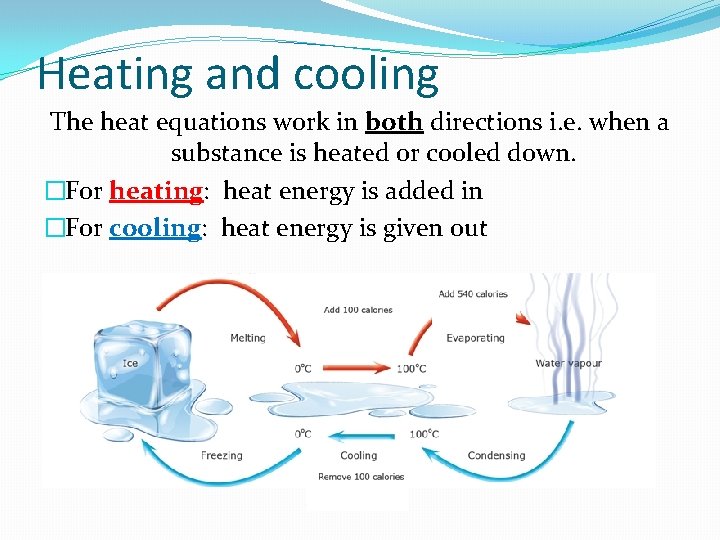
Heating and cooling The heat equations work in both directions i. e. when a substance is heated or cooled down. �For heating: heat energy is added in �For cooling: heat energy is given out h

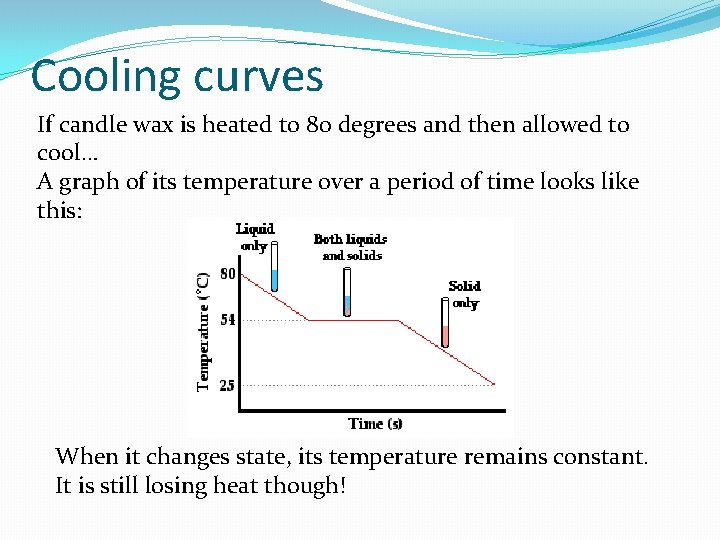
Cooling curves If candle wax is heated to 80 degrees and then allowed to cool… A graph of its temperature over a period of time looks like this: When it changes state, its temperature remains constant. It is still losing heat though!

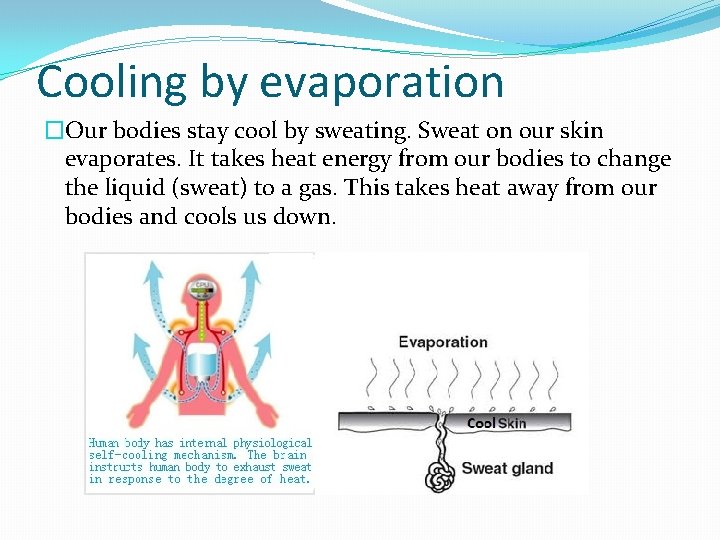
Cooling by evaporation �Our bodies stay cool by sweating. Sweat on our skin evaporates. It takes heat energy from our bodies to change the liquid (sweat) to a gas. This takes heat away from our bodies and cools us down.

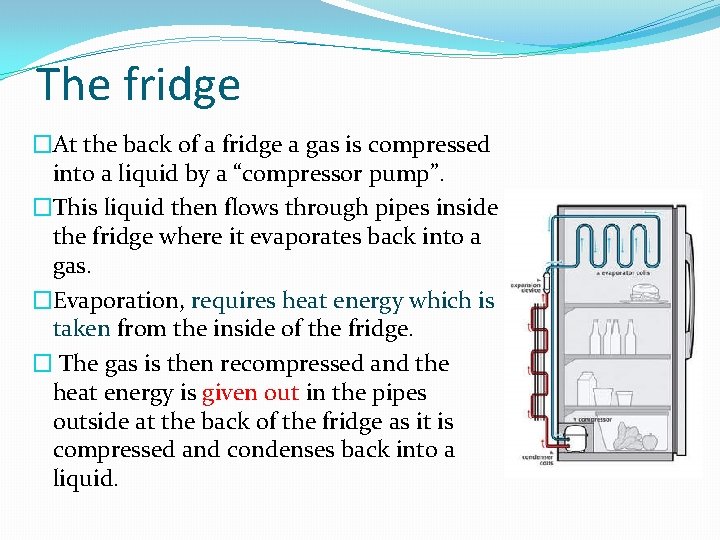
The fridge �At the back of a fridge a gas is compressed into a liquid by a “compressor pump”. �This liquid then flows through pipes inside the fridge where it evaporates back into a gas. �Evaporation, requires heat energy which is taken from the inside of the fridge. � The gas is then recompressed and the heat energy is given out in the pipes outside at the back of the fridge as it is compressed and condenses back into a liquid.