Chapter 10 Heat and Temperature Temperature Temperature is
















- Slides: 17

Chapter 10 Heat and Temperature

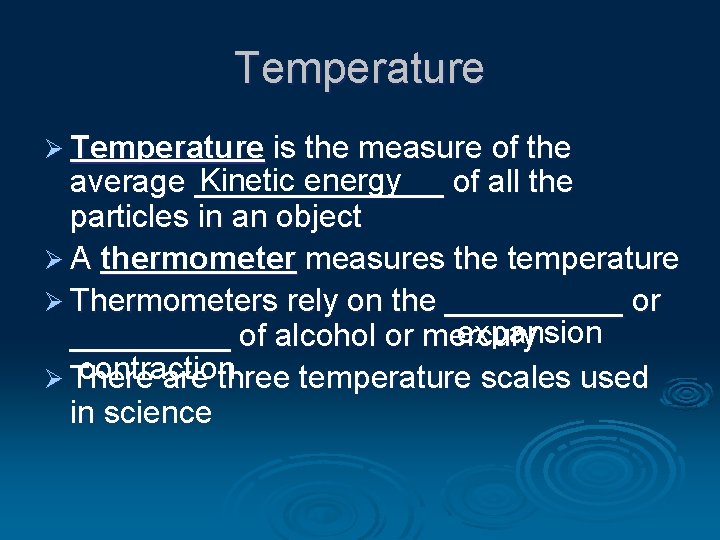
Temperature Ø Temperature is the measure of the Kinetic energy average _______ of all the particles in an object Ø A thermometer measures the temperature Ø Thermometers rely on the _____ or expansion _____ of alcohol or mercury contraction Ø There are three temperature scales used in science

Temperature Ø Fahrenheit scale (°F) 32 °F and boils at ____°F 212 Water freezes at ____ Celsius scale (°C) Ø Most countries use the _______ 0 100 l Water freezes at ___°C and boils at ___°C l Ø To convert temperatures we can use the following formulas: l l TF = 9/5 x TC + 32. 0 TC = 5/9 (TF – 32. 0)

Temperature Ø Kelvin scale (K) l l 0 Absolute zero = ______ K Figure 10 -5 P. 327 • Shows temperature scales side-to-side l l A unit of Kelvin is equal to a degree on the Celsius scale ______ We can convert Celsius to Kelvin: TK = TC + 273. 15

Energy Transfer Ø Why does ice melt? l l Energy is transferred from the surface to the ice Molecules in the ice speed up and their KE increases Ø Heat is the transfer of ______ energy between the particles of two objects due to a temperature difference __________

Energy Transfer Ø Conduction l Conduction is the transfer of energy as heat contact between two objects in _______ Ø Convection l Convection is the transfer of energy through a liquid or a gas a fluid…. _______ • Convection currents is the flow of a fluid due to expansion heated _____ • This is what allows buildings to cool and clouds to form l Land sea breezes

Energy Transfer Ø Radiation l l Radiation is the transfer of energy by electromagnetic waves ________ Fireplaces, the Sun, and the Earth transfer energy by radiation

Conductors Ø A conductor is any material through which energy can be transferred as heat Ø Gases are poor conductors of heat Ø Liquids conduct heat, but they are not very effective Ø Rubber and wood conduct heat about as well as a liquid Ø Metals are the best conductors

Insulators Ø An insulator is any material that is a poor energy conductor Ø An insulator reduces or stops unwanted energy transfer Ø Wood is a type of insulator, as well as plastic l This is the reason we use wooden and plastic cooking utensils



Specific Heat Ø Which becomes hotter faster? l l A plastic spoon, or a metal spoon? A pot of water on the stove or Lake Erie? Ø Specific heat refers to the amount of energy transferred as heat that will raise ______ 1 kg of a substance by the temperature of __ __ K 1. K J/ kg Ø Specific heat is expressed as ______

Specific Heat Ø Why is the temperature of Lake Erie low? Ø Why does it take all summer for the temperature of the lake to rise? Ø Equation: q = mcΔt l l q = energy (J) m = mass (kg) c = specific heat (J/kg K) t = temperature (K)

Specific Heat Ø How much energy must be transferred as heat to 420 kg of water in order to raise the water’s temperature from 25°C to 37°C? q = 420 kg x c=4186 q = mcΔt J/kg. K x 12 K q=? q = 21, 000 J m=420 kg Δt=37°C-25°C c=4186 J/kg. K

Using Heat Ø A _______ heating system is a device or process that transfers energy to a substance to raise the temperature of that substance Ø Your body transfers heat to your blood from food Ø When it is cold energy is transferred from skin to the air thus reducing the your ______, temperature of your skin

Using Heat Ø A ____ furnace uses warm water to radiate heat to the house Ø A ______ Solar heating system collects radiated energy from the sun to heat water Insulation minimizes unwanted energy Ø _____ transfers l Ex. fiberglass

Using Heat Ø A _______ cooling system is a device that transfers energy as heat out of an object to lower its temperature Ø Condensation and evaporation transfer energy to the surroundings l l Temperatures increase slightly Ex. Refrigerant and melting snow